In my clinical practice, the narrative is strikingly consistent. A patient sits across from me, exhausted and unable to lose weight. They are clutching a lab report that says their TSH is “normal.”
They take their Levothyroxine religiously every single morning. Yet, they feel no different than they did prior to diagnosis. The missing link in this clinical picture is rarely the thyroid gland itself.
Table of Contents
It is the metabolic environment in which the thyroid hormones are trying to function. The connection between sugar and hypothyroidism is a complex biochemical interplay. It extends far beyond simple calorie counting or lack of willpower.
It involves the pancreas, the liver, and a critical mechanism known as the insulin-thyroid feedback loop. When we consume high-glycemic foods, we trigger a cascade of hormonal events. These events can render thyroid medication ineffective and stall the body’s natural hormone production.
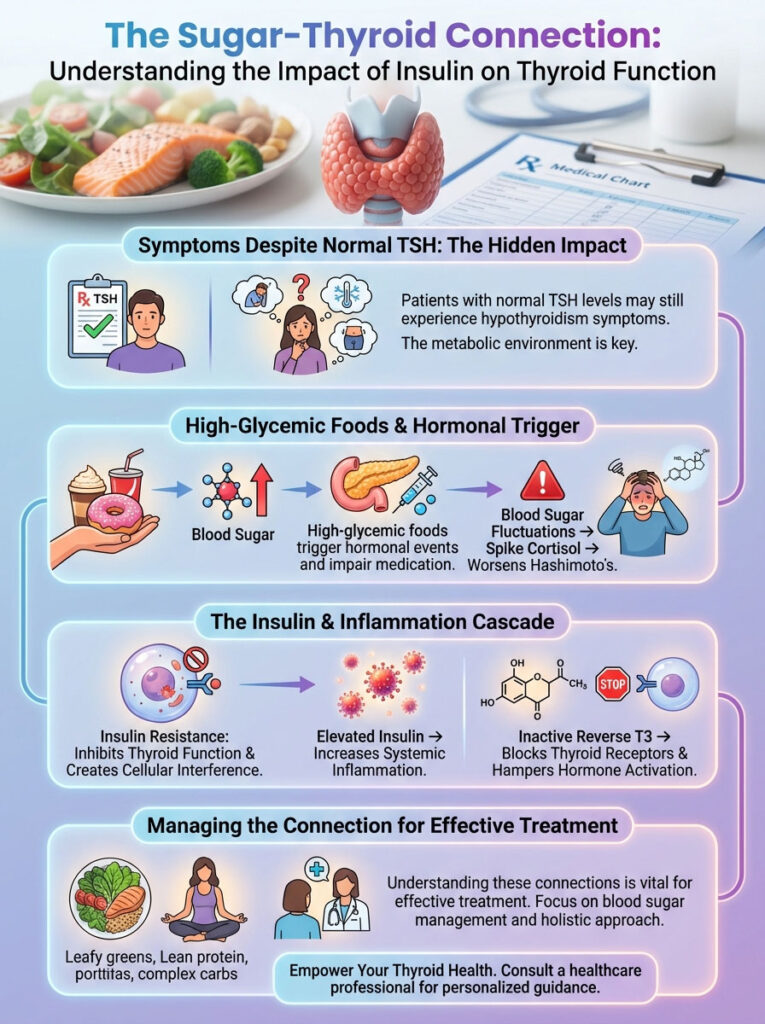
As an endocrinology researcher and clinician, I view insulin resistance not just as a precursor to diabetes. I see it as a direct inhibitor of thyroid function. High circulating insulin acts as cellular noise.
It drowns out the signals your thyroid sends to your metabolism. This article serves as a deep examination into the physiology of how sugar disrupts conversion. We will explore how it increases Reverse T3 and fuels the autoimmune fire of Hashimoto’s thyroiditis.
Quick Summary: The Sugar-Thyroid Connection
High sugar intake and subsequent hyperinsulinemia directly worsen hypothyroidism by impairing the enzymatic conversion of T4 to active T3. Elevated insulin levels trigger systemic inflammation and increase inflammatory cytokines like IL-6. These cytokines inhibit the 5′-deiodinase enzymes responsible for activating thyroid hormones in the liver and kidneys. Furthermore, insulin resistance creates a “metabolic trap” by promoting the production of Reverse T3 (rT3). This is an inactive hormone that blocks thyroid receptors. For patients with Hashimoto’s thyroiditis, blood sugar fluctuations spike cortisol. This can increase TPO antibody levels and exacerbate autoimmune attacks.
Key Statistics: The Metabolic Thyroid Crisis
- 60% of Conversion: The liver is responsible for converting 60% of inactive T4 into active T3; this process is heavily impaired by fructose-induced fatty liver.
- 5x Risk: Patients with subclinical hypothyroidism are up to 5 times more likely to develop insulin resistance compared to euthyroid individuals.
- 50% Overlap: Nearly half of patients with Hashimoto’s also present with reactive hypoglycemia or glucose dysregulation.
- < 5 uIU/mL: The optimal fasting insulin level for thyroid function, despite standard lab ranges allowing up to 25 uIU/mL.
- 90% of Cases: Hashimoto’s is the cause of 90% of hypothyroid cases in the US, making immune system regulation via blood sugar control vital.
- 30% Reduction: Studies show that correcting insulin resistance can reduce thyroid nodule volume by up to 30% in some patients.
The Physiology of the Insulin-Thyroid Feedback Loop
To understand why your diet impacts your thyroid medication, we must first look at the symbiotic relationship between organs. Specifically, we must examine the beta cells of the pancreas and the thyroid gland. In a healthy body, thyroid hormones regulate insulin clearance.
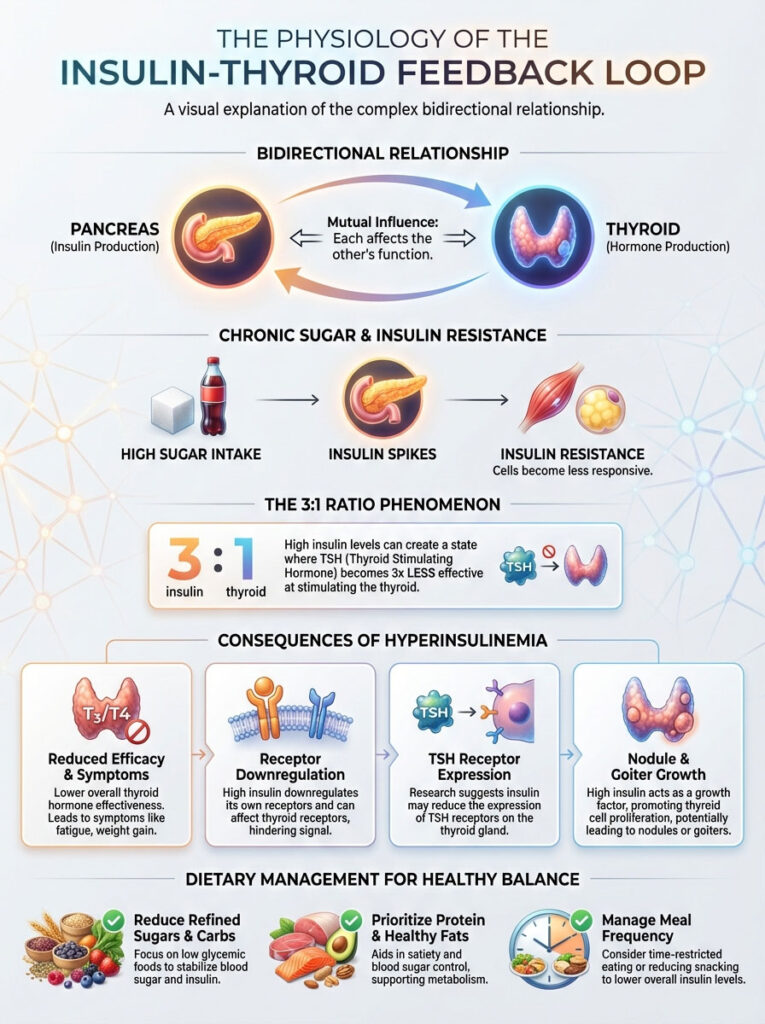
Conversely, insulin regulates thyroid hormone activity. It is a bidirectional street. However, this balance is fragile. When it is disrupted by chronic sugar consumption, we see the emergence of the “3:1 Ratio” phenomenon.
In clinical observations, patients with high fasting insulin often present with suboptimal Free T3 levels. This occurs even with normal TSH. This suggests that insulin resistance acts as a physiological barrier to thyroid hormone efficacy.
Hyperinsulinemia as an Endocrine Disruptor
Hyperinsulinemia refers to chronically high levels of insulin in the blood. This condition creates a state of “hormonal noise.” Think of your thyroid hormone as a radio signal.
Your cells are the receiver. High insulin is static interference. Even if the signal (T3) is strong, the cells cannot hear it clearly. This desensitization means you can have perfect blood levels of thyroid hormone.
Yet, you still suffer from hypothyroid symptoms. You may experience cold intolerance, hair loss, and crushing fatigue. The mechanism involves the downregulation of hormone receptors.
Research published in the Journal of Clinical Endocrinology & Metabolism has highlighted this modulation. It shows that insulin impacts the expression of thyrotropin (TSH) receptors. When insulin is consistently high, the thyroid gland itself may become less responsive.
It stops listening to the pituitary gland’s signals. This creates a sluggish response loop. Unfortunately, standard TSH testing often misses this dynamic entirely.
Insulin as a Growth Factor for Goiters
There is another dangerous aspect to high insulin levels. Insulin is an anabolic hormone. This means it promotes growth. In the context of the thyroid, this is not always beneficial.
Hyperinsulinemia can stimulate the proliferation of thyroid cells. This acts as a growth factor similar to IGF-1. This is a key driver in the formation of thyroid nodules and goiters.
If you have nodules that are growing despite normal TSH levels, look at your insulin. Reducing sugar intake is often the first line of defense to stop nodule enlargement. It removes the growth signal that is fueling the tissue expansion.
The Conversion Crisis: How Sugar Blocks T4 to T3
The most significant impact of sugar and hypothyroidism lies in the conversion pathway. Your thyroid gland primarily produces T4 (Thyroxine). T4 is a pro-hormone.
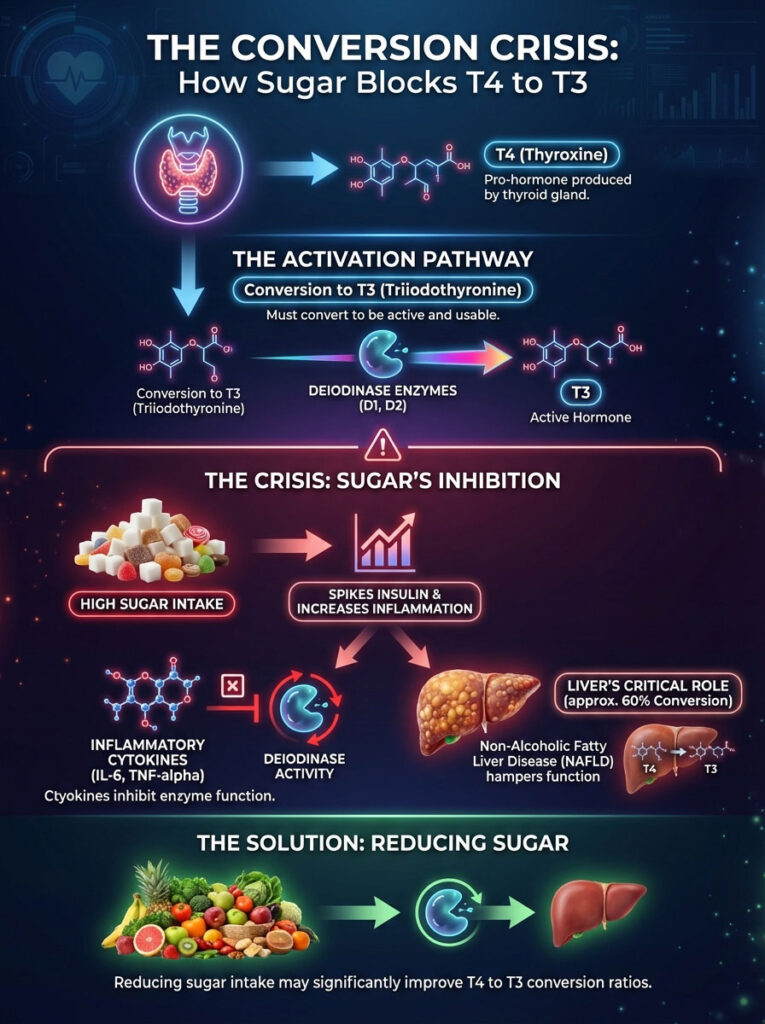
It is relatively inactive and serves as a storage unit. To produce energy, burn fat, and grow hair, your body must convert T4 into T3 (Triiodothyronine). This is the active “energy hormone.”
Without this conversion, T4 is useless. It is like having money in a bank account that is frozen. You have the assets, but you cannot spend them.
Understanding Deiodinase Enzymes
This conversion relies on specific enzymes called deiodinases. We are specifically concerned with types D1 and D2. These enzymes act like a switch.
They remove a specific iodine atom from T4 to turn it into T3. Here is the thing regarding these enzymes. They are incredibly sensitive to inflammation and oxidative stress.
When you consume a high-sugar diet, you spike insulin. This spike triggers the release of inflammatory cytokines. Specifically, we see rises in Interleukin-6 (IL-6) and TNF-alpha.
These cytokines downregulate deiodinase activity. Effectively, inflammation puts a padlock on the enzymes. You might be taking plenty of T4 (Levothyroxine).
But without functioning enzymes, it cannot be converted into the active form your body needs. This leads to a state of cellular hypothyroidism. Your blood work looks fine, but your cells are starving.
The Liver’s Role and NAFLD
We cannot discuss T4 to T3 conversion without discussing the liver. Approximately 60% of this conversion happens in the liver. Non-Alcoholic Fatty Liver Disease (NAFLD) is a massive epidemic.
It is driven primarily by excess fructose and sugar intake. Fructose is unique because it is metabolized almost exclusively by the liver. It does not require insulin for cellular entry like glucose does.
This sounds good, but it is actually dangerous. It puts a massive strain on liver mitochondria. This leads to rapid fat accumulation in liver cells.
When the liver becomes fatty or inflamed, its ability to perform enzymatic conversion drops precipitous. The hepatocytes are too busy managing fat toxicity to activate your thyroid hormone.
Expert Insight: If you have been diagnosed with “mild” fatty liver and have hypothyroidism, do not ignore it. Improving liver health through sugar reduction is often the single most effective way to improve your T4 to T3 conversion ratio.
The Reverse T3 (rT3) Trap
When the body is under stress, it engages a survival mechanism. This stress can be from starvation, trauma, or metabolic stress caused by insulin resistance. Instead of converting T4 into active T3, it converts it into Reverse T3 (rT3).
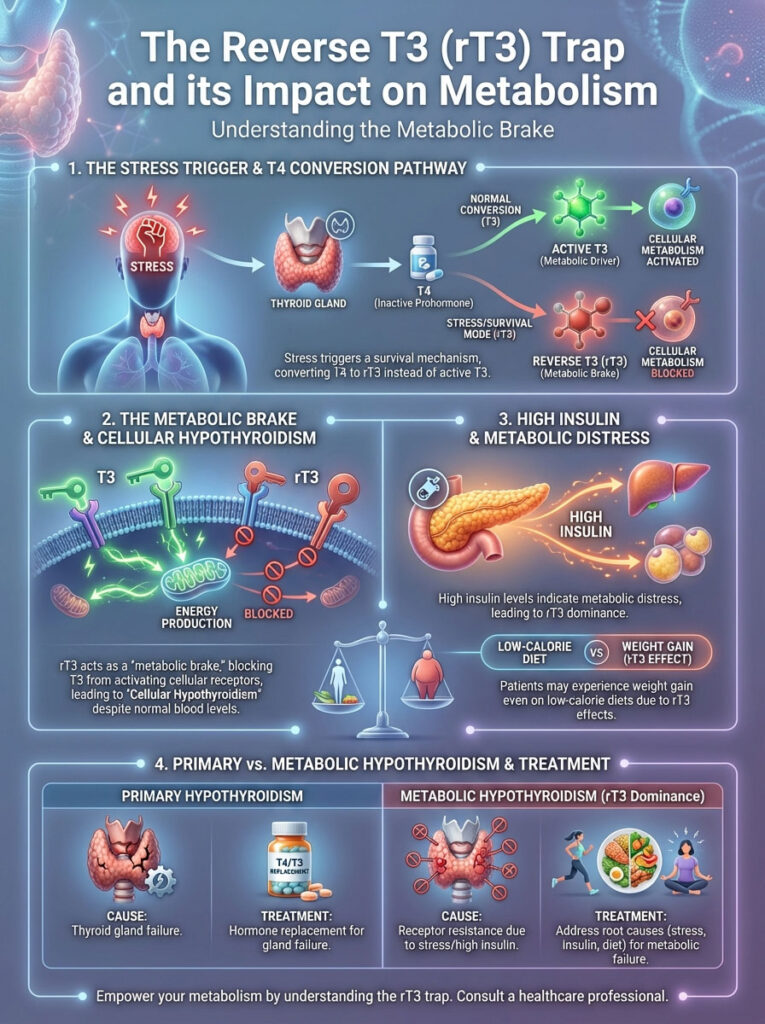
This is a protective mechanism designed to slow metabolism during famine. However, in the modern world, we are not in a famine. We are in a state of caloric excess but metabolic starvation.
Insulin’s Role in rT3 Dominance
Reverse T3 is the “metabolic brake.” It is a mirror image of T3. It fits into the same cellular receptors that T3 uses.
However, it does not activate them. It simply sits there. It blocks the receptor like a key broken off in a lock.
Active T3 cannot get in. Consequently, metabolism slows down. Hyperinsulinemia signals the body that it is in a state of metabolic distress.
To conserve energy, the body shifts conversion preference from Free T3 to Reverse T3. This leads to a state I call “Cellular Hypothyroidism.” The blood levels might look euthyroid (normal).
But the cells are starving for T3 because rT3 is blocking the receptors. This is why many patients gain weight even on low-calorie diets. Their metabolic rate has been chemically throttled by rT3.
Comparison Table: Primary vs. Metabolic Hypothyroidism
It is vital to distinguish between a gland failure and a metabolic failure. The treatments are different. Here is how they compare.
| Feature | Primary Hypothyroidism | Metabolic Hypothyroidism (Insulin-Induced) |
|---|---|---|
| Primary Cause | Thyroid gland failure or destruction | Peripheral conversion failure & receptor resistance |
| TSH Levels | Typically High (>4.5 mIU/L) | Often Normal (1.0–2.5 mIU/L) |
| Free T3 Levels | Low | Low or Low-Normal |
| Reverse T3 | Normal or Low | Elevated |
| Fasting Insulin | Variable | High (>10 uIU/mL) |
| Main Symptoms | Cold intolerance, hair loss, dry skin | Post-meal fatigue, sugar cravings, belly fat |
| Response to Meds | Symptoms improve with T4 | Symptoms persist despite T4 medication |
Hashimoto’s Thyroiditis and the Glycemic Rollercoaster
We must address the elephant in the room: Hashimoto’s Thyroiditis. This is an immune system issue. It is not just a thyroid issue.
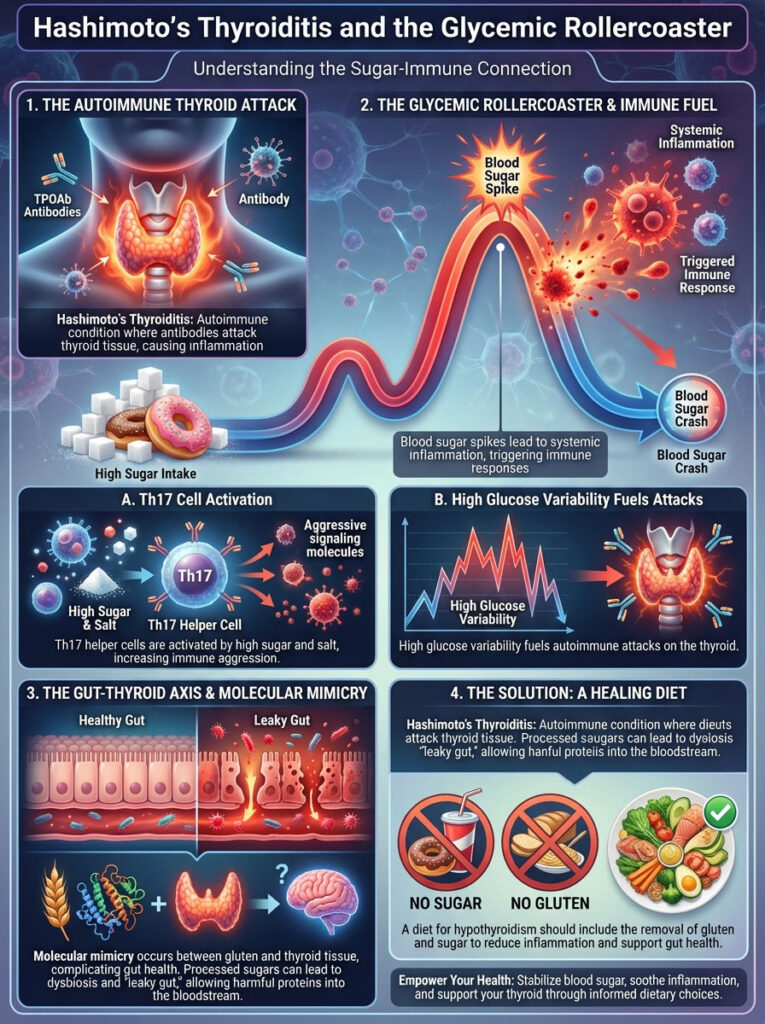
In Hashimoto’s, the body produces Thyroid Peroxidase Antibodies (TPOAb). These antibodies attack thyroid tissue. The connection between sugar and hypothyroidism here is mediated by the immune response.
The Sugar-Immune Response
Every time your blood sugar spikes significantly, it causes immediate systemic inflammation. This inflammation alerts the immune system. For a person with an autoimmune condition, this alert signal is dangerous.
It can trigger an aggressive attack on the thyroid gland. I tell my patients that you cannot lower your antibodies while fueling the fire. High glucose variability is like throwing gasoline on that fire.
This is driven by Th17 helper cells. These immune cells are highly active in autoimmune diseases. High salt and high sugar environments stimulate Th17 cells to become more aggressive.
Molecular Mimicry and Gut Health
Furthermore, we must consider molecular mimicry. Processed sugary foods often contain gluten. The molecular structure of gliadin (the protein in gluten) is strikingly similar to thyroid tissue.
Sugar feeds bad bacteria in the gut. This leads to dysbiosis and increased intestinal permeability, often called “leaky gut.” When the gut leaks, proteins like gluten and undigested sugars enter the bloodstream.
The immune system tags them as invaders. Because thyroid tissue looks like gluten, the immune system attacks the thyroid too. This is why a diet for hypothyroidism and insulin resistance often necessitates removing gluten alongside sugar.
The Adrenal-Thyroid-Pancreas Triad
The thyroid does not exist in a vacuum. It functions as part of a triad with the adrenals and the pancreas. This relationship is governed by the HPA Axis (Hypothalamus-Pituitary-Adrenal).
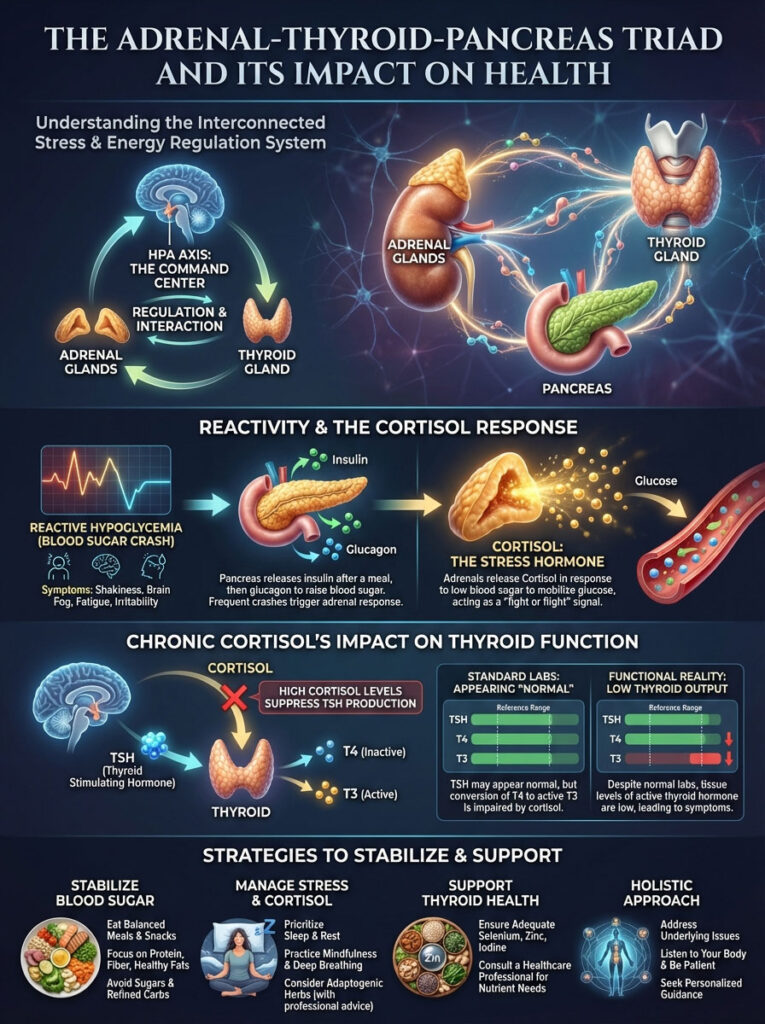
Reactive Hypoglycemia and Cortisol
Many patients with sugar and hypothyroidism issues experience reactive hypoglycemia. This is the “crash” that happens 2-3 hours after a high-carb meal. When blood sugar drops too low, the adrenal glands perceive a life-threatening emergency.
They release cortisol to liberate stored glucose from the liver. This is a survival mechanism. However, chronic cortisol release is disastrous for the thyroid.
Here is why this matters. High cortisol suppresses TSH production. It acts directly on the pituitary gland to say, “We are under stress, slow down the metabolism.”
This leads to a false “normal” on lab tests. Your TSH might look perfect because cortisol is suppressing it. But you feel terrible because your thyroid output is actually low.
To fix the thyroid, we must stabilize the blood sugar. This stops the cortisol spikes. It allows the pituitary to speak to the thyroid clearly again.
Metabolic Hypothyroidism: Recognizing the Symptoms
How do you know if insulin resistance is the culprit behind your thyroid woes? The symptoms of metabolic hypothyroidism are distinct. They differ from classic hypothyroidism in subtle ways.
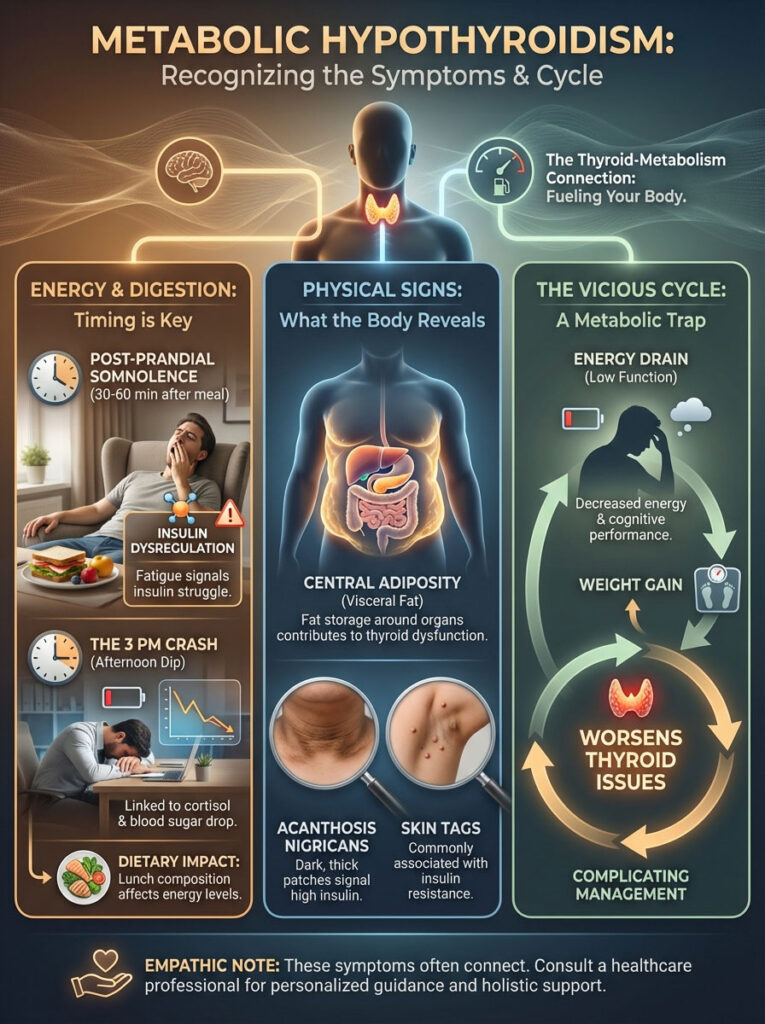
Post-Prandial Somnolence
The most telling sign is fatigue that occurs 30 to 60 minutes after a meal. If you eat lunch and feel an overwhelming need to nap, pay attention. This is a sign of insulin dysregulation.
Your body is expending massive amounts of energy trying to process the glucose load. It diverts resources away from cognitive function. A healthy metabolism should feel energized after eating, not sedated.
The 3 PM Crash
Thyroid patients often hit a wall in the mid-afternoon. This is rarely just “tiredness.” It is usually the intersection of a circadian dip in cortisol and a drop in blood sugar.
If you rely on caffeine or sugar to get through this window, you are perpetuating the cycle. You are causing adrenal stress and poor T4 to T3 conversion. Breaking this cycle requires a change in your lunch composition.
Central Adiposity
Insulin resistance causes fat storage specifically around the organs. This is known as visceral fat. This is not just cosmetic.
Visceral fat is metabolically active tissue. It produces its own inflammatory cytokines called adipokines. These adipokines further inhibit thyroid function.
It becomes a vicious cycle. Low thyroid causes weight gain. That specific type of weight gain further suppresses the thyroid.
Acanthosis Nigricans and Skin Tags
Look at your skin. Do you have darker, velvety patches on your neck, armpits, or groin? This is Acanthosis Nigricans.
It is a hallmark sign of high insulin levels. Similarly, an abundance of skin tags is often linked to insulin resistance. These physical signs often appear years before blood sugar reaches diabetic levels.
Essential Lab Tests for the Metabolic Thyroid Patient
If you suspect sugar and hypothyroidism are linked in your case, standard labs are insufficient. You need a metabolic assessment. Here is the specific panel to request from your physician.

- Fasting Insulin: This is the gold standard for early detection. A normal fasting glucose does not rule out insulin issues. Target: < 5 uIU/mL.
- HbA1c: A marker of your average blood sugar over the last 3 months. Target: 4.8% – 5.2%.
- Free T3 and Free T4: Essential to calculate the conversion ratio. You want to see if T4 is high but T3 is low.
- Reverse T3: To check for the “metabolic brake.” If this is elevated, insulin resistance or inflammation is likely present.
- TPO and Tg Antibodies: To monitor autoimmune activity in Hashimoto’s Thyroiditis.
- Lipid Panel (Triglycerides): High triglycerides are a surrogate marker for high insulin. If they are over 100 mg/dL, suspect sugar issues.
- GGT (Gamma-Glutamyl Transferase): This liver enzyme is often elevated in fatty liver disease. It can indicate if the liver is struggling with conversion.
Dietary Strategies to Restore Sensitivity
The goal of a diet for hypothyroidism and insulin resistance is not just weight loss. It is hormonal restoration. We must lower the insulin demand to allow the deiodinase enzymes to recover.

The Low Glycemic Load Approach
We focus on “Glycemic Load” rather than just “Glycemic Index.” Glycemic Load accounts for the portion size. It also accounts for the actual impact on blood sugar.
Low glycemic foods for thyroid health should be rich in micronutrients. We are not just avoiding sugar. We are adding value.
Protein Anchoring
A simple yet powerful strategy is “Protein Anchoring.” Never eat a carbohydrate alone. Always start your meal with protein.
Protein stimulates the release of glucagon. This hormone opposes insulin. By eating protein first, you blunt the subsequent insulin spike from carbohydrates.
Studies show this can reduce the glucose spike by up to 30%. This protects your T4 to T3 conversion pathways. It also keeps you fuller for longer.
The Fruit Controversy
Is fruit bad for hypothyroidism? Not all fruit is created equal. High-sugar fruits like mangoes, grapes, or dried fruit can spike insulin significantly.
However, we do not want to eliminate fruit entirely. Berries are low glycemic foods for thyroid health. Blueberries, raspberries, and blackberries are rich in antioxidants.
These antioxidants protect the thyroid gland from oxidative stress. The key is moderation and timing. Eat fruit with fat or protein, never alone.
Comparison Table: High Glycemic vs. Thyroid-Optimized Foods
Making the switch doesn’t mean eating cardboard. It means choosing foods that burn cleaner. Here is a guide to swapping.
| Food Category | High Glycemic (Avoid) | Thyroid-Optimized (Include) | Impact on Thyroid |
|---|---|---|---|
| Grains | White bread, instant oats, pasta | Quinoa, buckwheat, black rice | Complex carbs stabilize insulin preventing cortisol spikes |
| Fruits | Dried fruits, fruit juices, canned fruit | Blueberries, raspberries, avocado | Berries provide antioxidants to protect the thyroid gland |
| Proteins | Processed deli meats, breaded meats | Wild salmon, grass-fed beef, eggs | Selenium/Zinc in quality proteins support conversion |
| Fats | Trans fats, vegetable oils (soybean) | Coconut oil, olive oil, ghee | Healthy fats are required for hormonal cell wall integrity |
| Sweeteners | High Fructose Corn Syrup, Agave | Stevia, Monk fruit, small amounts of raw honey | Natural sweeteners minimize the insulin surge |
Navigating Sweeteners: What is Safe?
When patients quit sugar, they often turn to artificial sweeteners. This can be a mistake. We need to be selective.

Artificial Sweeteners and the Gut
Substances like Aspartame and Sucralose may have zero calories. However, they can negatively impact the gut microbiome. Since 20% of T4 to T3 conversion happens in the gut, we must protect our bacteria.
Research suggests that some artificial sweeteners can induce glucose intolerance. This defeats the purpose of quitting sugar. They trick the brain into expecting calories that never arrive, which can stimulate appetite.
Better Alternatives
What is the best sweetener for thyroid patients? Monk fruit and pure Stevia are generally best. They do not trigger an insulin response.
They are natural extracts. However, ensure they are not cut with fillers like dextrose or maltodextrin. Read the labels carefully.
Natural raw honey can be tolerated by some patients in small amounts. It contains enzymes and minerals. However, it is still sugar, so use it sparingly.
Supplements and Therapeutics for Insulin-Thyroid Balance
While food is the foundation, targeted supplementation can accelerate healing. We want to repair the insulin-thyroid feedback loop. Always consult your physician before starting new protocols.
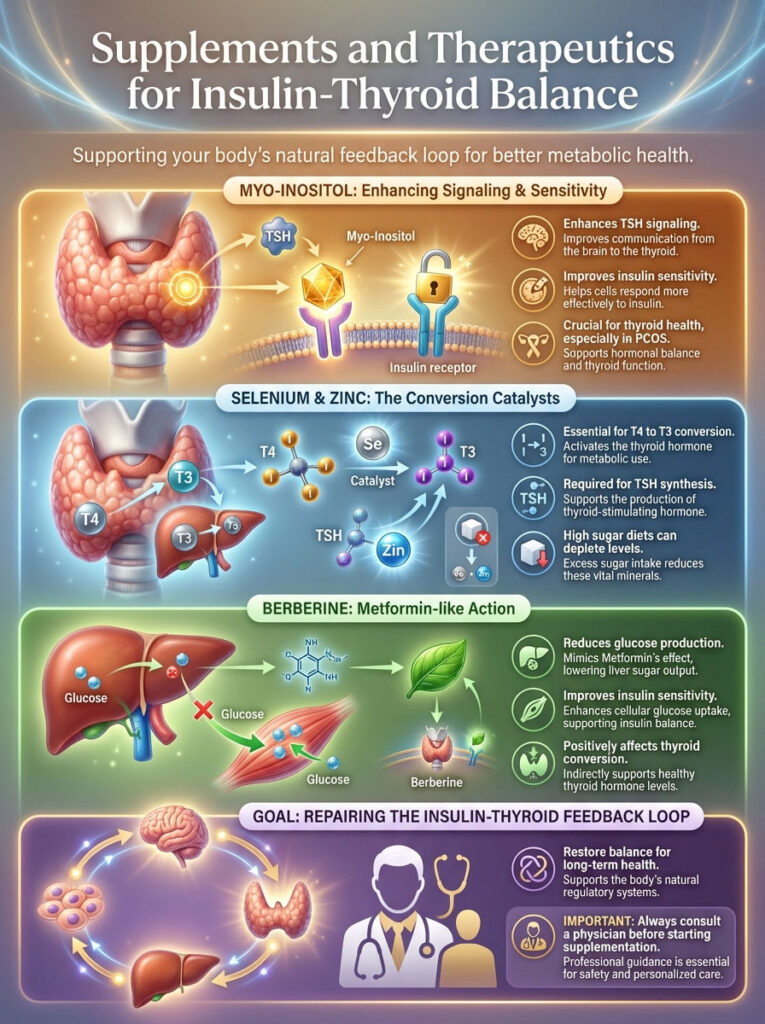
Myo-Inositol: The Signal Booster
Myo-inositol is often discussed in the context of PCOS. But it is critical for thyroid patients as well. Research indicates that Myo-inositol acts as a second messenger for TSH.
It improves the sensitivity of the thyroid gland to TSH signaling. Simultaneously, it improves insulin sensitivity. It helps fix the “deafness” of the cells.
Studies have shown that combining Myo-inositol with Selenium can lower TSH levels more effectively than Selenium alone. It addresses the root cause of the resistance.
Selenium and Zinc: The Conversion Crew
These are the “conversion co-factors.” The deiodinase enzymes that convert T4 to T3 are selenium-dependent. They are actually called selenoproteins.
Without adequate selenium, T4 to T3 conversion halts. Zinc is equally vital for the synthesis of TSH. High sugar diets often deplete these minerals through increased urinary excretion.
If you have high blood sugar, you are likely peeing out your zinc. Replenishing these stores is non-negotiable for recovery.
Berberine: Nature’s Metformin
Berberine is a powerful botanical found in plants like Oregon Grape and Goldenseal. It has been shown to activate AMPK. This is a metabolic master switch.
It functions similarly to Metformin. It improves insulin sensitivity and reduces glucose production in the liver. For patients with insulin resistance and high Reverse T3, Berberine can be a game-changer.
It helps clear the glucose from the blood. This lowers the insulin requirement. Consequently, inflammation drops, and thyroid conversion improves.
Lifestyle Factors: Sleep and Stress
You cannot out-supplement a bad lifestyle. Sleep and stress management are physiological pillars. They directly impact how your body handles sugar.
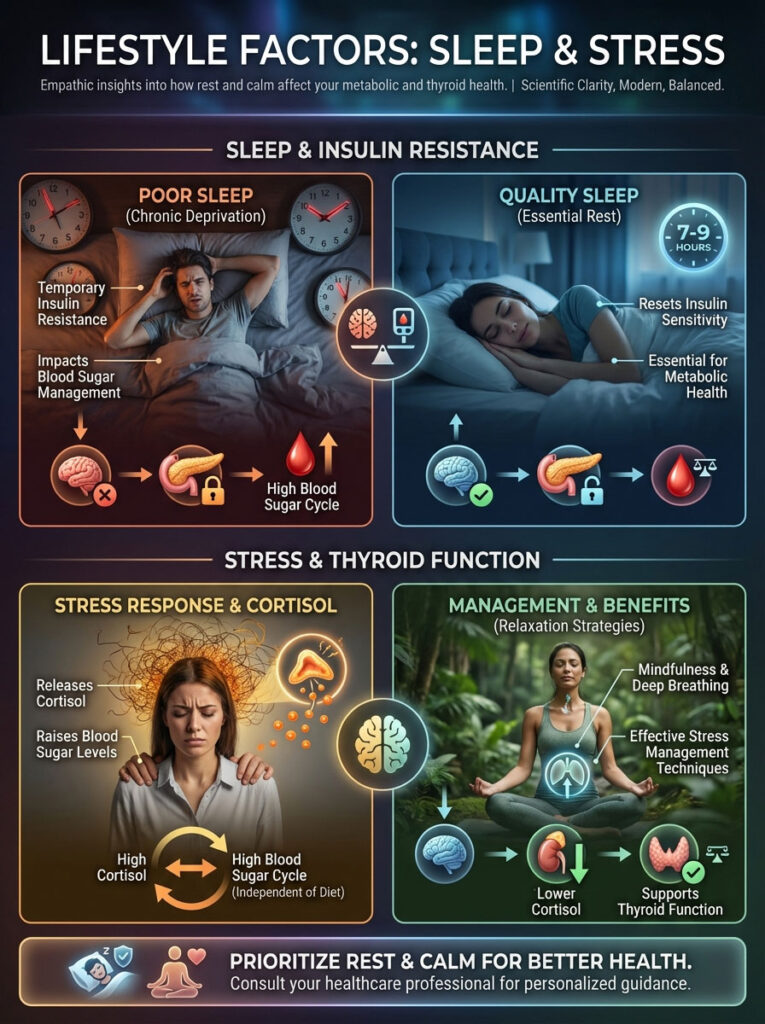
The Sleep-Sugar Connection
One night of poor sleep can induce temporary insulin resistance. If you are chronically sleep-deprived, your cells become resistant to insulin. This causes your pancreas to pump out more.
This happens even if your diet is perfect. Prioritizing 7-9 hours of quality sleep is a thyroid therapy. It resets the insulin sensitivity for the next day.
Stress and Glucose
Does stress affect how your body handles sugar? Absolutely. Stress releases cortisol.
Cortisol releases stored glucose into the bloodstream. This creates a cycle of high blood sugar and insulin resistance even if you aren’t eating sweets. You could be fasting and still have high blood sugar due to stress.
Mindfulness, deep breathing, and gentle movement are not just “nice to haves.” They are biochemical interventions. They lower cortisol, which lowers glucose, which helps the thyroid.
Summary & Key Takeaways
The journey to resolving thyroid symptoms is rarely a straight line. However, the connection between sugar and hypothyroidism provides a clear map for intervention. You cannot fix the thyroid without addressing the insulin.
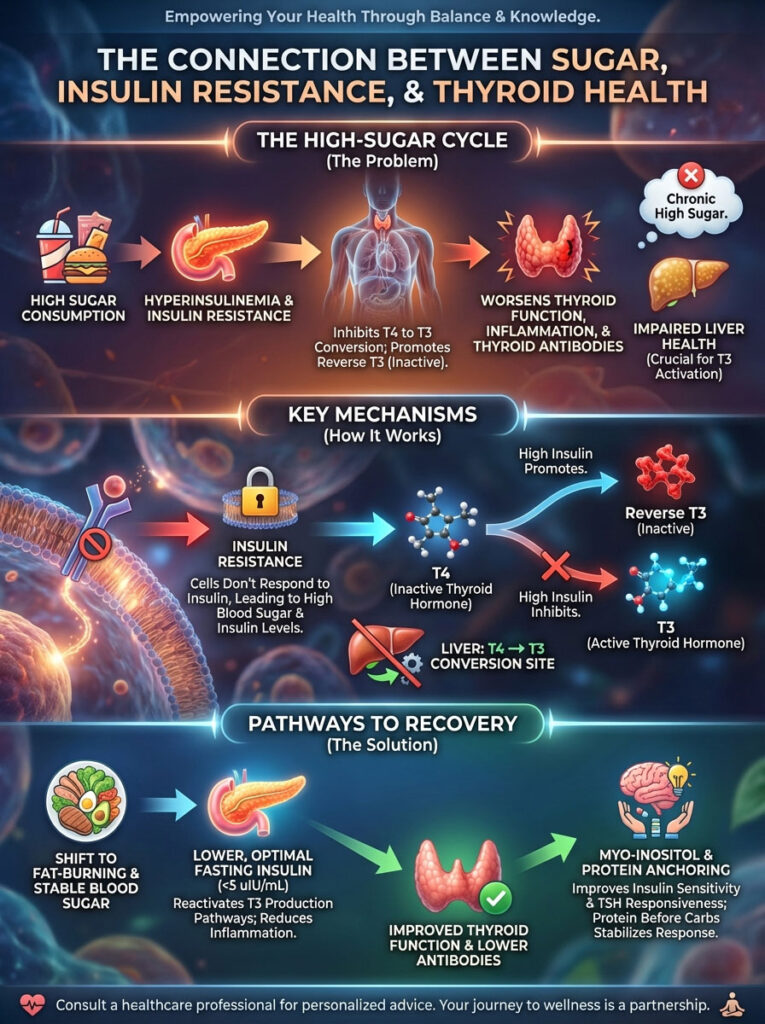
The physiological reality is undeniable. Insulin resistance acts as a potent inhibitor of T4 to T3 conversion. It is also a primary promoter of Reverse T3.
By shifting your metabolic state from sugar-burning to fat-burning, you reactivate enzymatic pathways. These pathways favor T3 production. This reduces the inflammatory burden that drives Hashimoto’s thyroiditis.
It also lowers Thyroid Peroxidase Antibodies. I encourage you to look beyond the TSH. Advocate for a full metabolic assessment.
Consult with a functional practitioner who can interpret these advanced labs. Let them guide you toward a diet for hypothyroidism and insulin resistance. This is the path to restoring your vitality.
Frequently Asked Questions
How does sugar intake interfere with thyroid hormone conversion?
High sugar consumption triggers hyperinsulinemia, which releases inflammatory cytokines like IL-6 and TNF-alpha. These cytokines inhibit the 5\’-deiodinase enzymes responsible for converting inactive T4 into active T3, primarily in the liver and kidneys, leading to cellular hypothyroidism.
Why do my hypothyroid symptoms persist even with a normal TSH?
This is often due to metabolic hypothyroidism where insulin resistance creates a barrier to hormone efficacy at the cellular level. Even if your pituitary gland is satisfied, your cells may be “deaf” to the thyroid signal or blocked by high levels of Reverse T3 caused by metabolic stress.
What is the relationship between insulin resistance and Reverse T3?
Insulin resistance acts as a metabolic stressor that signals the body to conserve energy by shifting the conversion of T4 away from active T3 and toward Reverse T3 (rT3). Reverse T3 is an inactive isomer that binds to and blocks thyroid receptors, effectively acting as a metabolic brake that slows your fat-burning potential.
Can high insulin levels cause thyroid nodules or goiters to grow?
Yes, insulin is a potent anabolic hormone that promotes cellular proliferation and growth. Chronically high insulin acts as a growth factor, similar to IGF-1, which can stimulate the enlargement of thyroid tissue and the formation of nodules even when TSH levels are within the normal range.
How does sugar consumption affect Hashimoto’s antibody levels?
Spikes in blood glucose cause systemic inflammation and stimulate Th17 immune cells, which are known to intensify autoimmune attacks on the thyroid gland. Maintaining stable blood sugar is essential to lowering Thyroid Peroxidase (TPO) antibodies and preventing further destruction of thyroid tissue.
What are the optimal fasting insulin levels for someone with hypothyroidism?
While standard laboratory ranges often allow for fasting insulin up to 25 uIU/mL, clinical excellence for thyroid function requires a level below 5 uIU/mL. Levels above this threshold typically indicate enough insulin resistance to impair the conversion of T4 to active T3 and drive weight gain.
Why is the liver so important for thyroid patients who eat high-sugar diets?
The liver is responsible for approximately 60% of thyroid hormone activation; however, excess fructose leads to Non-Alcoholic Fatty Liver Disease (NAFLD). When the liver is congested with fat, its ability to perform enzymatic conversion drops significantly, leaving the body in a state of energy deficiency.
What is Protein Anchoring and how does it help thyroid health?
Protein anchoring is the dietary strategy of consuming protein before carbohydrates to stimulate glucagon release and blunt the subsequent insulin spike. This technique reduces glucose variability, which protects the sensitive deiodinase enzymes and prevents the cortisol spikes that suppress thyroid function.
Are artificial sweeteners like sucralose safe for thyroid patients?
I generally advise against artificial sweeteners as they can disrupt the gut microbiome, where 20% of thyroid conversion occurs via the “gut-thyroid axis.” Furthermore, some sweeteners may induce glucose intolerance, which defeats the purpose of avoiding sugar for metabolic health.
How does Myo-inositol improve both insulin sensitivity and thyroid function?
Myo-inositol acts as a second messenger for TSH, making the thyroid gland more responsive to hormonal signaling from the pituitary. Simultaneously, it improves insulin receptor sensitivity, making it a dual-action therapeutic for patients struggling with both Hashimoto\’s and insulin resistance.
Can reactive hypoglycemia cause a false normal TSH reading?
Yes, the blood sugar “crash” associated with reactive hypoglycemia triggers a survival release of cortisol. Since chronic cortisol elevation suppresses TSH production, your lab results may show a TSH level that looks “perfect” while your actual thyroid output is insufficient for your metabolic needs.
What role does Berberine play in managing metabolic hypothyroidism?
Berberine activates the AMPK pathway, which improves insulin sensitivity and reduces glucose production in the liver. By lowering the insulin requirement, it helps clear the “hormonal noise” that prevents T3 from reaching cellular receptors, effectively boosting the metabolic rate.
Disclaimer
This article is for informational purposes only and does not constitute medical advice. The connection between sugar and hypothyroidism is complex; always consult a qualified healthcare professional or endocrinologist before making changes to your medication, diet, or supplement regimen.
References
- American Thyroid Association – thyroid.org – Guidelines on thyroid function tests and the impact of systemic illness on hormone levels.
- Journal of Clinical Endocrinology & Metabolism – academic.oup.com/jcem – Peer-reviewed research on the impact of insulin resistance on TSH receptor expression.
- Endocrine Society – endocrine.org – Clinical practice resources regarding the interplay between metabolic syndrome and thyroid dysfunction.
- PubMed / NCBI – ncbi.nlm.nih.gov – Study on the effects of Myo-inositol and Selenium on subclinical hypothyroidism and autoimmune markers.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) – niddk.nih.gov – Data on insulin resistance, NAFLD, and their systemic endocrine effects.
- Journal of Thyroid Research – hindawi.com/journals/jtr – Research regarding deiodinase enzyme activity and the inhibitors of T4 to T3 conversion.