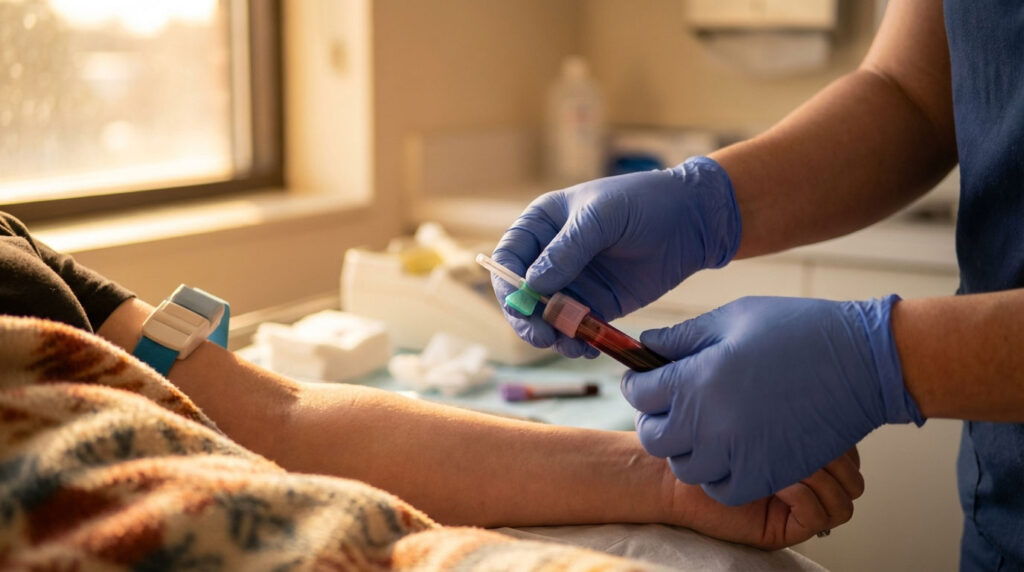
A Simple Blood Draw Can Now Screen for Colon Cancer. Here’s What You Need to Know.
One in three Americans who should be screened for colorectal cancer simply don’t do it. That’s roughly 50 million people, according to the American Cancer Society. And the reasons are predictable: colonoscopy prep is miserable, stool tests feel unpleasant, and life just gets in the way.
But as of early 2026, there’s a new option sitting on the table at your doctor’s office. It’s a blood test. Just a standard blood draw. No fasting. No prep. No sedation. And it could catch colon cancer before you ever feel a single symptom.
The First FDA-Approved Blood Test for Colon Cancer Screening
The test is called Shield, made by Guardant Health. The FDA approved it in July 2024, making it the first and only blood test cleared as a primary screening option for colorectal cancer in average-risk adults ages 45 and older. That approval was based on the ECLIPSE trial, one of the largest cancer screening studies ever conducted, which enrolled more than 20,000 people across 200 clinical trial sites in 34 states.
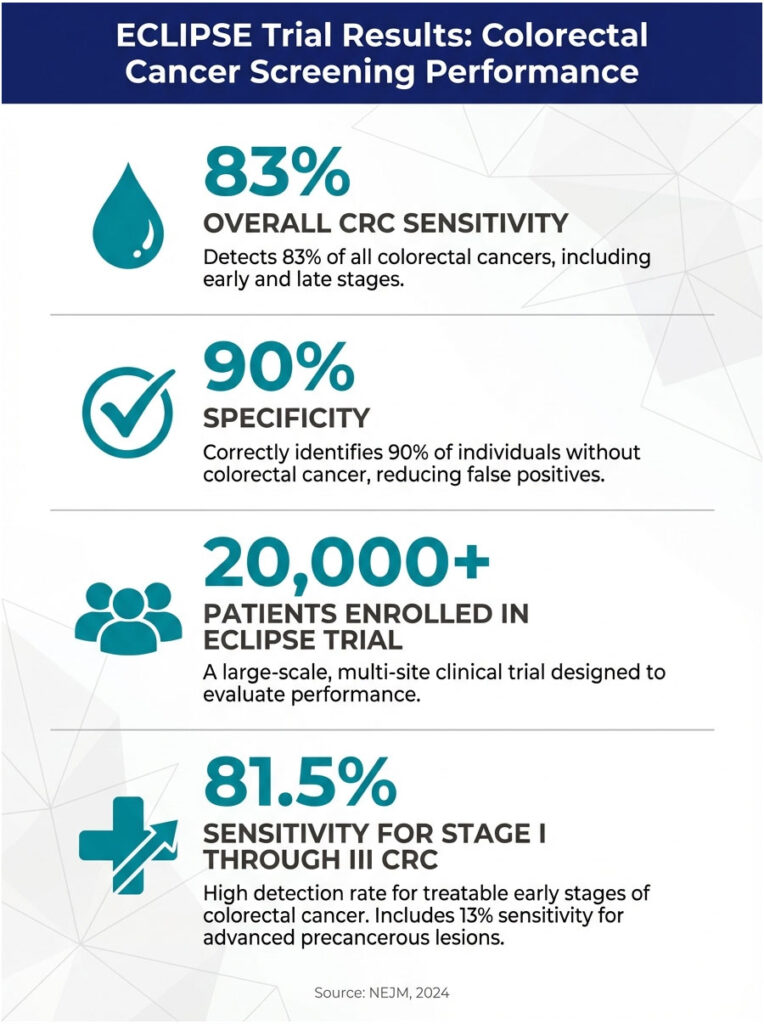
The results, published in The New England Journal of Medicine, showed Shield detected 83% of colorectal cancers with 90% specificity. To put that in context, other non-invasive screening tests (like stool-based options) have sensitivity ranges of roughly 74% to 94%, depending on the specific test and version. Shield’s sensitivity for detecting stage I through III cancers (the curable stages) was 81.5%.
Here’s where the numbers get important. The study included 7,861 people who met all criteria. Of those with colonoscopy-confirmed colorectal cancer, 83.1% had a positive Shield test. The test was less effective at catching precancerous polyps, detecting only about 13% of advanced precancerous lesions. That’s a significant limitation, and it matters.
Why This Matters for Millions of Americans
Colorectal cancer is the second leading cause of cancer death in the United States when you combine men and women. The American Cancer Society estimates about 158,850 new colorectal cancer cases and 55,230 deaths from the disease in 2026. And here’s the part that makes doctors lose sleep: when caught early (before it spreads), the five-year survival rate is 91%. Catch it late, and the picture changes dramatically.
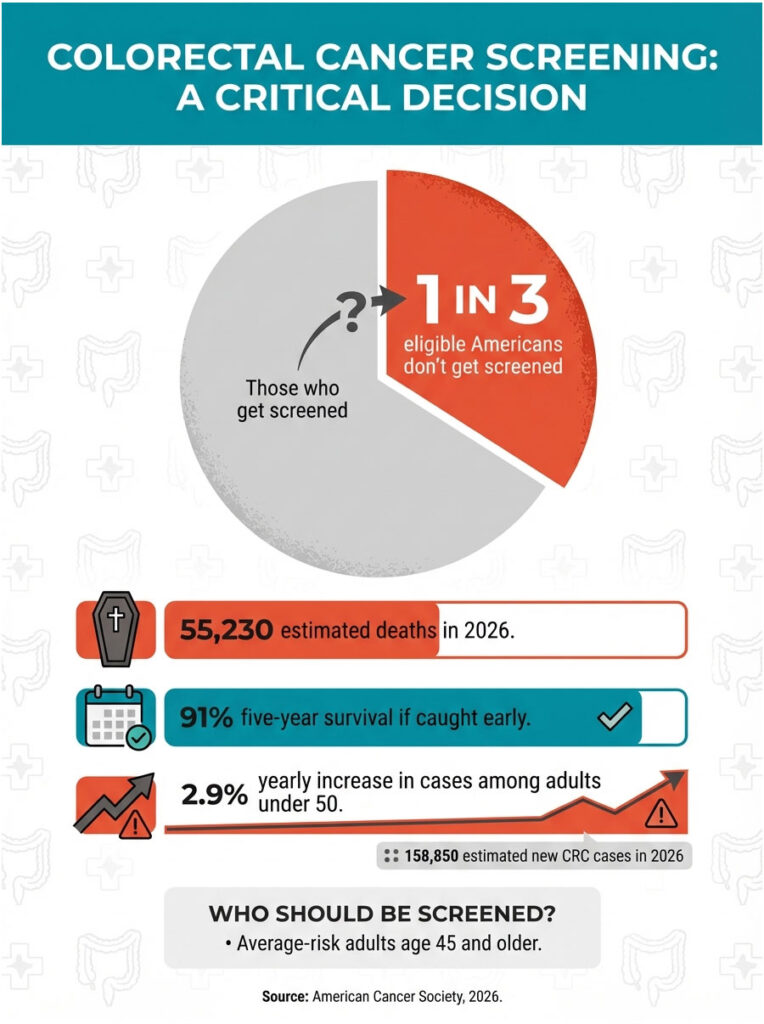
The screening gap is the problem. About 35% of eligible Americans aren’t up to date on their screening. Among adults aged 45 to 49, the age group most recently added to screening guidelines, the rates are even lower. Black Americans are 20% more likely to be diagnosed with colorectal cancer and 40% more likely to die from it. Veterans face higher incidence rates too.
The logic behind a blood test is straightforward: if people won’t do the colonoscopy or the stool test, maybe they’ll roll up their sleeve. And the early data supports that idea. Since Shield launched as a lab-developed test in 2022, more than 90% of patients who were prescribed it actually completed it. One study found that colorectal cancer screening rates tripled among adults who had previously declined all other screening options when they were offered Shield instead.
What the Experts Are Saying
“Over 50 million eligible Americans do not get recommended screenings for colorectal cancer, partly because current screening methods are inconvenient or unpleasant,” said AmirAli Talasaz, Guardant Health co-CEO.
“Having a blood-based test for people to take during routine doctor’s visits could be an opportunity to help more people be screened,” said William M. Grady, MD, medical director of the Gastrointestinal Cancer Prevention Program at Fred Hutchinson Cancer Center and the corresponding author of the ECLIPSE study published in NEJM. Dr. Grady is a paid member of Guardant’s scientific advisory board.
“Any screening is better than no screening,” said Cathy Eng, MD, a professor of medicine at Vanderbilt University Medical Center, who was not involved in the ECLIPSE study. She noted that while the Shield test has limitations, its convenience could help close the screening gap in communities that need it most.
Not everyone is sold, though. Experts at Memorial Sloan Kettering Cancer Center have raised concerns. Robin Mendelsohn, MD, co-director of MSK’s Center for Young Onset Colorectal and Gastrointestinal Cancers, has said her team doesn’t recommend the Shield test as a screening tool, pointing to its lower detection rate for precancerous polyps.
The American Gastroenterological Association has also urged caution, noting that blood tests “cannot be recommended to replace established CRC screening methods” based on current data.
How to Actually Get the Shield Blood Test
So if you’re interested, here’s what the process looks like in practice.
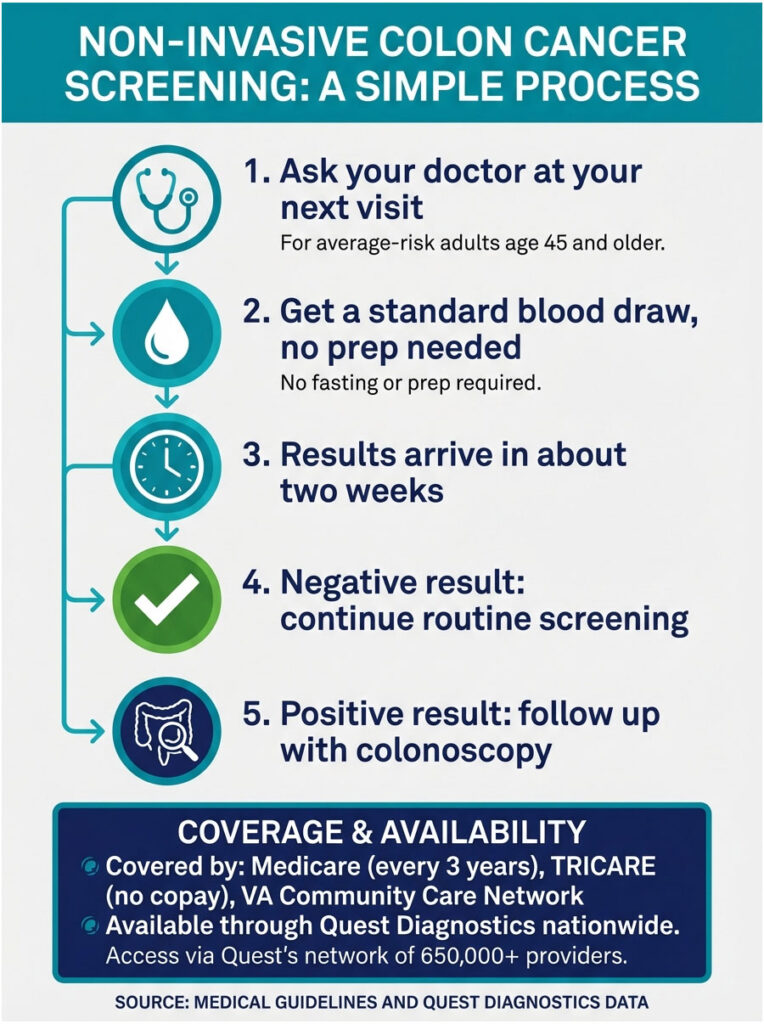
Start by asking your doctor at your next routine visit. Any prescribing healthcare provider can order the Shield test. You don’t need to see a specialist. The test requires just a standard blood draw, the same kind you’d get for a cholesterol check or a metabolic panel. No fasting required, no bowel prep, no time off work. Your blood is sent to Guardant Health’s lab, and results typically come back within about two weeks.
As of early 2026, access has expanded significantly. Quest Diagnostics, which serves roughly 650,000 healthcare providers across the country, began offering Shield through its existing ordering system in the first quarter of 2026. That means if your doctor already uses Quest for lab work, ordering Shield can be as simple as checking a box. Medicare covers the test for eligible adults every three years. TRICARE now covers it for active-duty military service members and their families with no copay. The VA Community Care Network also provides coverage.
If the test comes back negative, you continue with routine screening at the recommended intervals. A negative result doesn’t guarantee you’re cancer-free, and you should still follow up with your doctor about your individual risk. If the test comes back positive, it means there may be signs of colorectal cancer or advanced precancerous tissue. The next step is a diagnostic colonoscopy to confirm or rule out the finding.
The Shield test is designed for people at average risk for colorectal cancer, age 45 and older. It’s not intended for people with a personal or family history of colorectal cancer, inflammatory bowel disease, or hereditary cancer syndromes. Those individuals should follow their doctor’s advice on more targeted screening.
The Honest Limitations You Should Know
No screening test is perfect, and Shield is no exception. Here are the things worth understanding before you decide.
The test caught 83% of colorectal cancers in the ECLIPSE trial, which means it missed about 17%. For stage I cancers specifically, the detection rate was lower, around 55% to 65%. That’s a meaningful gap compared to colonoscopy, which remains the gold standard for finding both cancers and precancerous polyps.
The biggest concern is precancerous polyps. Shield detected only about 13% of advanced precancerous lesions. Colonoscopy can find and remove these polyps before they ever become cancer, which is something no blood test or stool test can do. That’s a key distinction: colonoscopy can prevent cancer, while Shield and stool tests can detect it.
The ECLIPSE study was funded by Guardant Health, and several study authors have financial ties to the company. That doesn’t invalidate the results, but it’s worth noting for transparency.
The NCCN (National Comprehensive Cancer Network) added the blood-based test to its updated screening guidelines in June 2025, but only for average-risk individuals who can’t or won’t use other screening methods. The U.S. Preventive Services Task Force and the American Cancer Society have not yet included it in their recommendations.
This is an observational screening study, not a randomized controlled trial measuring whether the test reduces colorectal cancer deaths. That kind of long-term outcomes data will take years to collect.
The Bottom Line

For the 50 million Americans who skip colon cancer screening entirely, a simple blood test could be the difference between catching cancer early and catching it too late. Shield isn’t a replacement for colonoscopy. But for people who won’t get screened any other way, it’s a real option that’s now widely available, covered by insurance, and as easy as rolling up your sleeve at a routine checkup.
If you’re 45 or older and haven’t been screened, talk to your doctor. The best screening test is the one you actually do.
MEDICAL DISCLAIMER
This article is meant to keep you informed, not to replace your doctor’s advice. Before making any decisions about cancer screening, it’s always a good idea to talk with a healthcare provider who knows your medical history and your individual risk factors. Everyone’s situation is different, and the right screening method for one person may not be the right choice for another. If you’re experiencing symptoms like blood in your stool, unexplained weight loss, or changes in bowel habits, please seek medical attention right away.
REFERENCES AND SOURCES
[1] Chung DC, Gray DM, Singh H, et al. “A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening.” The New England Journal of Medicine, March 14, 2024. DOI: 10.1056/NEJMoa2304714. https://www.nejm.org/doi/abs/10.1056/NEJMoa2304714
[2] National Cancer Institute. “Shield Blood Test Approved for Colorectal Cancer Screening.” Cancer Currents Blog, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2024/shield-blood-test-colorectal-cancer-screening
[3] American Cancer Society. “Colorectal Cancer Statistics: How Common Is Colorectal Cancer?” 2026. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
[4] Guardant Health. “Shield Blood Test for Colorectal Cancer Screening Now Available for U.S. Military Members and Families.” Press Release, January 8, 2026. https://investors.guardanthealth.com/press-releases/press-releases/2026/Guardant-Healths-Shield-Blood-Test-for-Colorectal-Cancer-Screening-Now-Available-for-U-S–Military-Members-and-Families/default.aspx
[5] Guardant Health and Quest Diagnostics. “Strategic Collaboration to Broaden Access to Shield Blood-based Screening Test.” Press Release, 2025. https://investors.guardanthealth.com/press-releases/press-releases/2025/Guardant-Health-and-Quest-Diagnostics-Announce-Strategic-Collaboration-to-Broaden-Access-to-Guardants-Shield-Blood-based-Screening-Test-in-the-United-States/default.aspx
[6] Medicare.gov. “Blood-based Biomarker Tests (Screening) for Colorectal Cancer.” https://www.medicare.gov/coverage/blood-based-biomarker-tests-screening-for-colorectal-cancer
[7] American Gastroenterological Association. “FDA Approves First Blood Test for Colorectal Cancer.” July 2024. https://gastro.org/news/fda-approves-first-blood-test-for-colorectal-cancer/
[8] Memorial Sloan Kettering Cancer Center. “Is Shield a Good Colorectal Cancer Screening Blood Test?” August 2025. https://www.mskcc.org/news/is-shield-good-colorectal-cancer-screening-blood-test