Maybe you popped a couple of Advil for a splitting headache last night. Or maybe you have been on prescription-strength Motrin for weeks to manage chronic back pain. Now a pre-employment drug screening or a random workplace test is around the corner, and one thought keeps nagging at you: will ibuprofen show up on a drug test?
Table of Contents
It is a surprisingly common worry, and it deserves a straight answer.
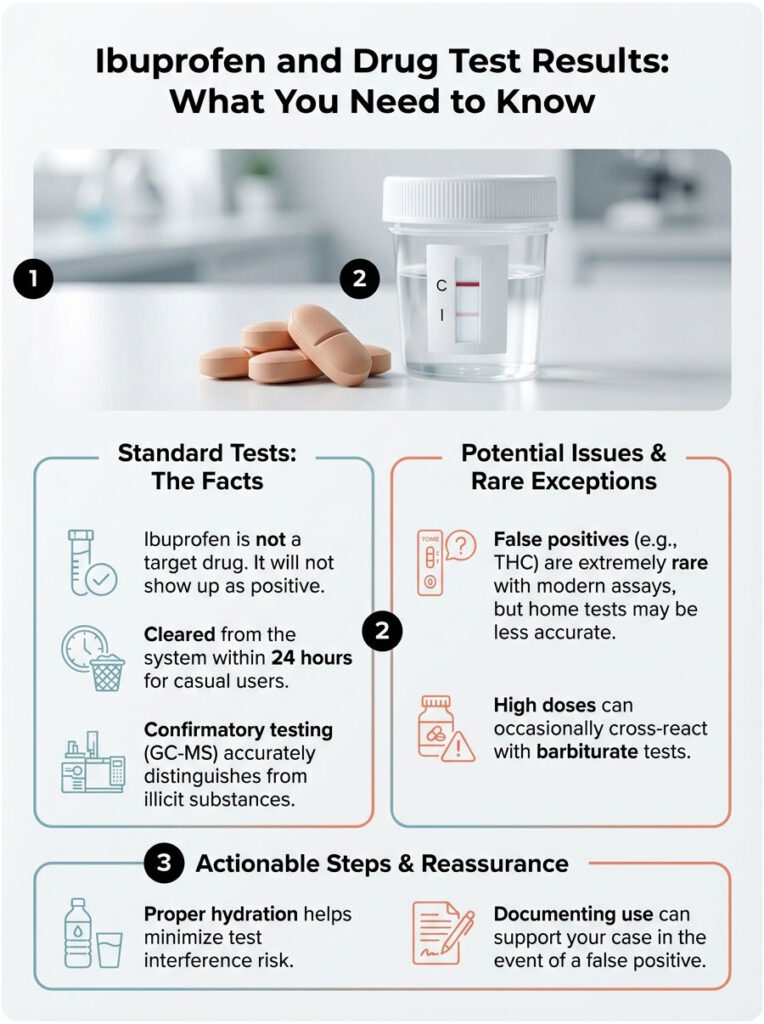
Quick Answer: Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID), not a controlled substance. Standard drug panels never specifically test for it. In rare cases, high doses can trigger a false positive for THC (marijuana) or barbiturates on the initial immunoassay screen due to cross-reactivity. When that happens, a confirmatory GC-MS test is required. That advanced test identifies the substance as ibuprofen, and the final report comes back “Negative.”

As a clinical toxicologist who has reviewed thousands of screening results, I see this anxiety regularly. People worry about over-the-counter medications more than they should, largely because they do not understand how the testing process actually works. And that is fair. The science of drug testing has come a long way since the early days of high error rates.
This article walks through the biochemistry behind false positives, the specific metabolites involved, and the safeguards, like the Medical Review Officer (MRO), that are designed to make sure your lawful pain management never costs you a job.
Key Statistics and Data Points
- Cross-Reactivity Rate: Modern immunoassays show a cross-reactivity rate of less than 0.01% for ibuprofen.
- False Positive Risk: Historically highest for cannabinoids (THC) and barbiturates.
- Clearance Time: Ibuprofen has a half-life of roughly 2 hours. About 99% is cleared within 24 hours.
- Screening Cutoff: Standard THC screens trigger at 50 ng/mL.
- Confirmation Accuracy: GC-MS testing is considered 100% accurate at distinguishing NSAIDs from illicit drugs.
- Annual Volume: Over 50 million workplace drug tests are performed each year in the United States.
How Drug Screening Actually Works
To understand why an ibuprofen false positive happens, you need to know how the test itself operates. Most workplace screenings happen in two phases: the initial screen and the confirmation. The mistake almost always occurs in phase one.
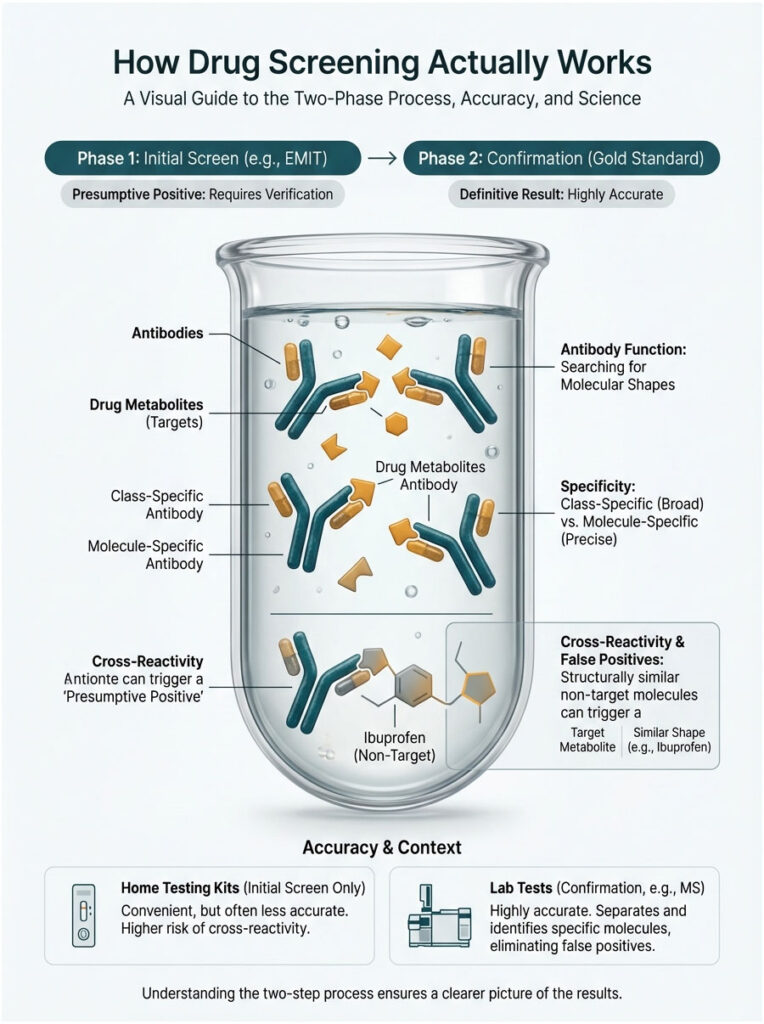
The Immunoassay (EMIT) Screen
The first line of defense in nearly every urine drug test is the Enzyme-Multiplied Immunoassay Technique (EMIT). Think of it as casting a wide net. The lab designs antibodies, which are microscopic proteins shaped like a lock. The drug metabolites are the key.
When urine hits the testing reagent, these antibodies search for specific molecular shapes. If one finds a match for its “lock,” it binds and triggers a chemical reaction, usually a color change. The machine reads that as a “presumptive positive.”
Here is the catch. These antibodies are class-specific, not molecule-specific. They are scanning for a general structure, not a single compound. For marijuana, they look for the carboxylic acid structure found in THC metabolites. That distinction is where the specificity problem begins.
Cross-Reactivity: Why False Positives Happen
Immunoassay cross-reactivity is the main reason behind false alarms. Antibodies are smart, but they are not perfect. If a legal medication carries a molecular structure that looks roughly 80% like an illegal drug, the antibody can get confused and bind to it anyway.
Ibuprofen (isobutylphenylpropionic acid) shares certain structural similarities with target metabolites of other drugs. In the 1990s, lab reagents were far less sophisticated. Taking 1,200mg of ibuprofen back then could easily trigger a false positive for THC. Today, reagent manufacturers have refined their antibodies considerably. They are much more selective. Still, biology is messy. At high concentrations, ibuprofen metabolites can occasionally mimic the polarity and shape of illicit substances, fooling the initial screen.
Expert Note: The “instant” cup tests and dipsticks sold at pharmacies have much higher rates of drug test interference than the high-tech analyzers used by LabCorp or Quest Diagnostics. If you test positive on a home kit after taking Advil, do not panic. It is almost certainly a chemistry error, not a true positive.
Can Ibuprofen Actually Trigger a False Positive?
We know it is technically possible. But how likely is it in a modern lab? The answer depends on two things: which drug class is being tested and how much ibuprofen you took.
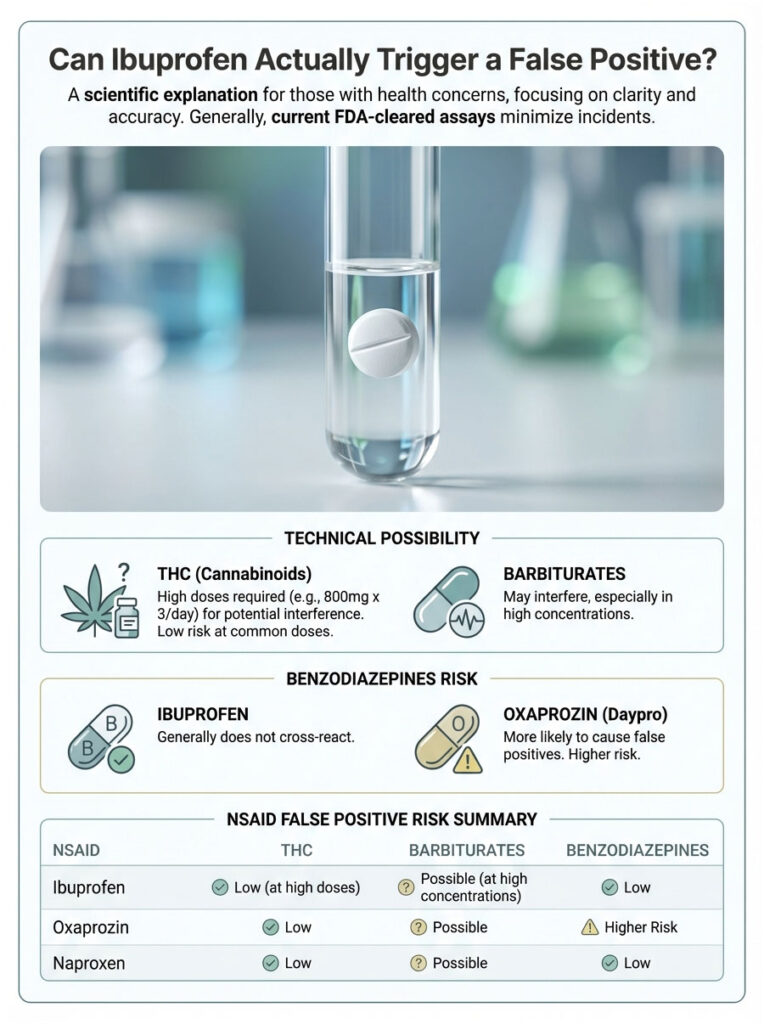
Ibuprofen and Cannabinoids (THC)
The most well-documented ibuprofen false positive involves marijuana. The primary marijuana metabolite labs test for is 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid. The key word in that long name is “carboxylic acid.” Ibuprofen is also a propionic acid derivative.
Under certain pH conditions in urine, the ibuprofen metabolite can bind to the cannabinoid antibody, especially with older EMIT assays. However, with current FDA-cleared assays used in certified labs, this is extremely rare. You would generally need to be taking chronic, high prescription doses, something like 800mg three times a day, to create enough interference to cross the 50 ng/mL cutoff.
Ibuprofen and Barbiturates
A less talked-about but real risk is a false positive for barbiturates. Barbiturates are older sedatives like phenobarbital, and they still appear on 10-panel screens. Both ibuprofen and naproxen (Aleve) have been linked to false positives in this category.
The interference mechanism is similar. The enzymatic reaction gets triggered by a high concentration of the NSAID, which is most often seen in patients treating conditions like rheumatoid arthritis who maintain elevated serum levels of anti-inflammatories.
Ibuprofen and Benzodiazepines
Some patients worry that Advil might flag as Xanax or Valium. Generally, ibuprofen does not cross-react with the benzodiazepine assay. That particular error is usually tied to a different NSAID called Oxaprozin (Daypro). Individual biochemistry varies, and in extremely rare cases involving liver enzyme differences, unpredictable cross-reactions can occur. But for the vast majority of people, ibuprofen will not trigger a benzo positive.
NSAID False Positive Comparison
| NSAID / Medication | Potential False Positive For | Risk Level | Mechanism of Interference |
| Ibuprofen (Advil/Motrin) | Cannabinoids (THC), Barbiturates | Low | Structural similarity to carboxylic acid metabolites |
| Naproxen (Aleve) | Barbiturates | Moderate | High protein binding and half-life extension |
| Oxaprozin (Daypro) | Benzodiazepines | High | Chemical structure mimics benzo rings |
| Diclofenac (Voltaren) | Cannabinoids (THC) | Very Low | Rare interference in specific assays |
The Gold Standard: How Confirmatory Testing Protects You
This is the section that should put your mind at ease. If your sample screens positive because of drug test interference, it does not mean you failed. It just means the sample needs further investigation. That is where confirmatory testing comes in.
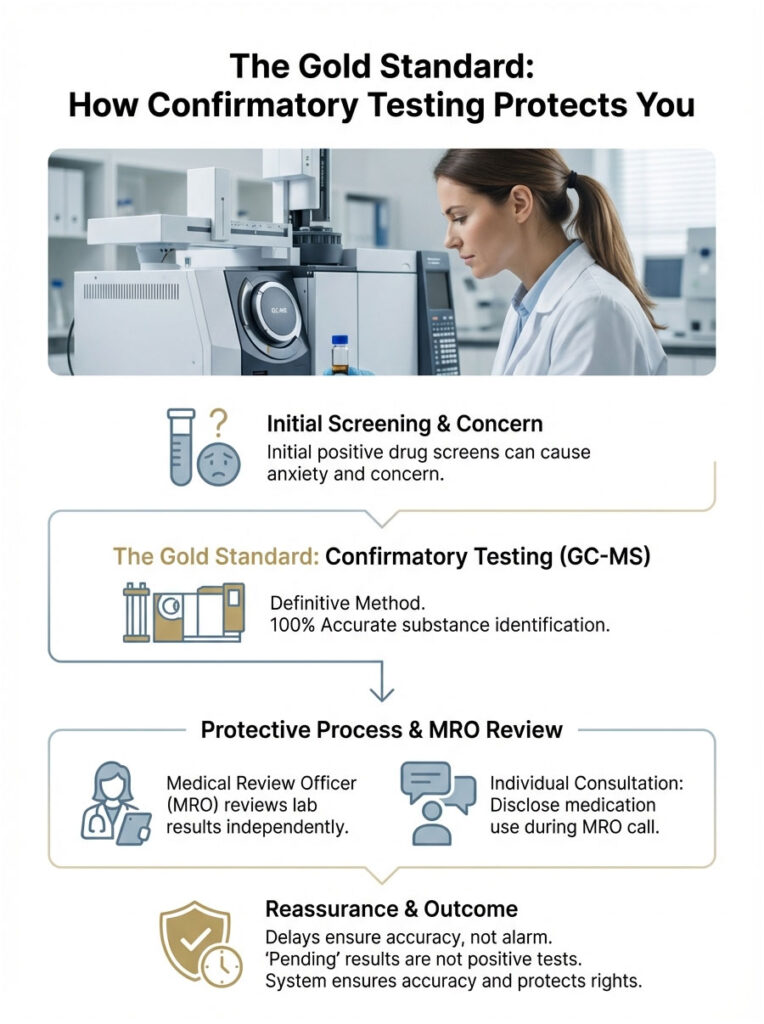
Gas Chromatography-Mass Spectrometry (GC-MS)
If the EMIT screen is the “net,” GC-MS is the microscope. The lab takes a fresh portion of your urine sample and injects it into a gas chromatograph. The machine vaporizes the urine and sends the vapor through a long, coiled tube. Different molecules travel at different speeds. When they exit the tube, an electron beam shatters them into fragments. The mass spectrometer then weighs those fragments.
This is molecular fingerprinting. The atomic weight and fragmentation pattern of ibuprofen are completely unique and distinct from those of THC or barbiturates. GC-MS cannot be fooled by ibuprofen. It distinguishes the atomic mass with 100% accuracy. If the initial screen was a false positive, GC-MS will find zero traces of the illicit drug, and the result gets updated to “Negative.”
The Role of the Medical Review Officer (MRO)
The Medical Review Officer is a licensed physician who reviews lab results. They serve as the impartial gatekeeper between the lab and the employer.
If a confirmed positive comes through, the MRO is required to contact you and ask what medications you are taking. This is your chance to disclose ibuprofen use. However, because GC-MS is so precise, the MRO rarely sees a positive result caused by Advil. The lab equipment rules it out long before it reaches their desk.
Why the Waiting Period Creates Stress
Most of the anxiety comes from the delay. An initial screen gives instant results. Confirmation testing takes 48 to 72 hours. During that window, the employer might be told the result is “Pending.” That waiting game is where the stress lives. But “Pending” does not mean “Positive.” It simply means the system is working as designed to protect you.
How Long Does Ibuprofen Stay in Your System?
Understanding how long ibuprofen remains detectable can help you plan around a test, especially if you want to avoid the hassle of a false alarm altogether.
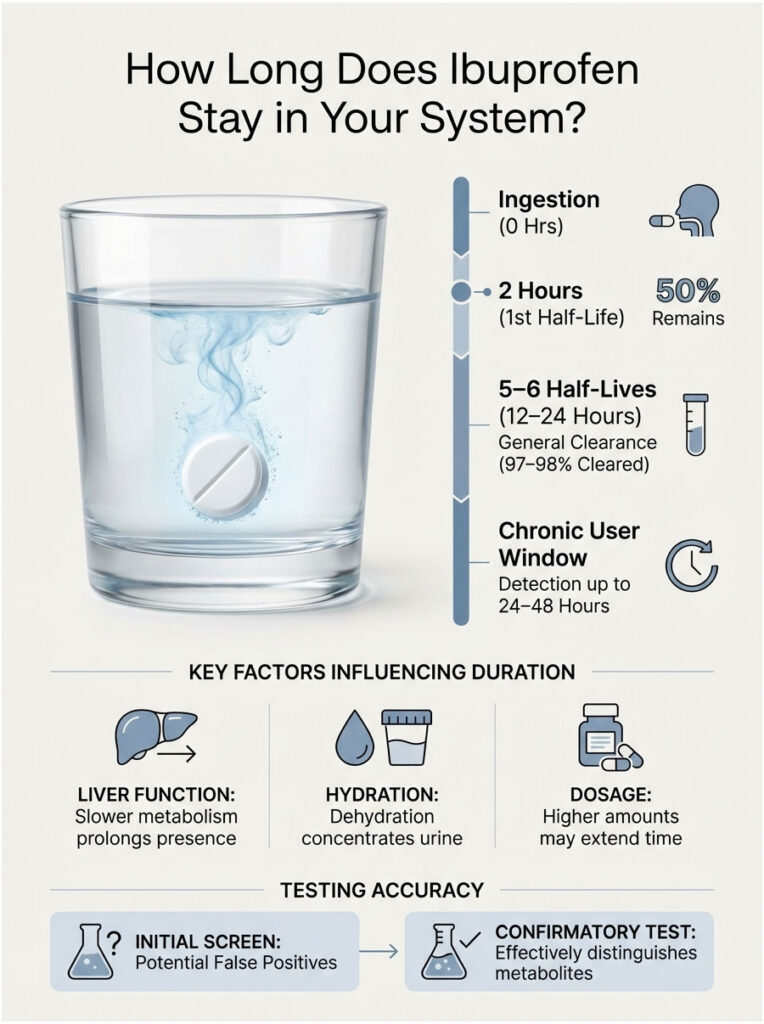
The Half-Life of Ibuprofen
The half-life of ibuprofen is approximately 2 hours. In toxicology, a drug is generally considered “cleared” after 5 to 6 half-lives. Quick math: 2 hours times 6 equals 12 hours. For someone taking a standard 200mg or 400mg dose, the metabolites are largely gone within 12 to 24 hours. There is very little risk of buildup unless you have kidney failure.
What Affects How Long It Stays
Several factors can extend this window. Chronic users taking 800mg three times daily for inflammation may have a steady-state drug level. In those cases, the detection window can stretch to 24-48 hours.
Your liver function matters too. The CYP2C9 enzyme handles ibuprofen metabolism. If you are a “slow metabolizer,” the drug lingers longer, which slightly increases the theoretical risk of cross-reactivity.
Hydration plays a role as well. Dehydration concentrates your urine, raising the specific gravity and the concentration of all metabolites, including ibuprofen. If you are dehydrated and took a high dose of Advil, the concentration in the urine sample will be higher, making it slightly more likely to trip a sensitive screen.
Testing Phases at a Glance
| Testing Phase | Methodology | THC Cutoff | Specificity | Can Ibuprofen Trigger It? |
| Initial Screen | Immunoassay (EMIT/ELISA) | 50 ng/mL | Low (class-specific) | Yes (rarely, as false positive) |
| Confirmatory | GC-MS / LC-MS/MS | 15 ng/mL | High (molecule-specific) | No (distinguishes metabolites) |
Workplace and Federal Drug Testing Guidelines (SAMHSA)
Drug testing rules are not arbitrary. They are governed by strict federal standards, primarily set by the Substance Abuse and Mental Health Services Administration (SAMHSA).
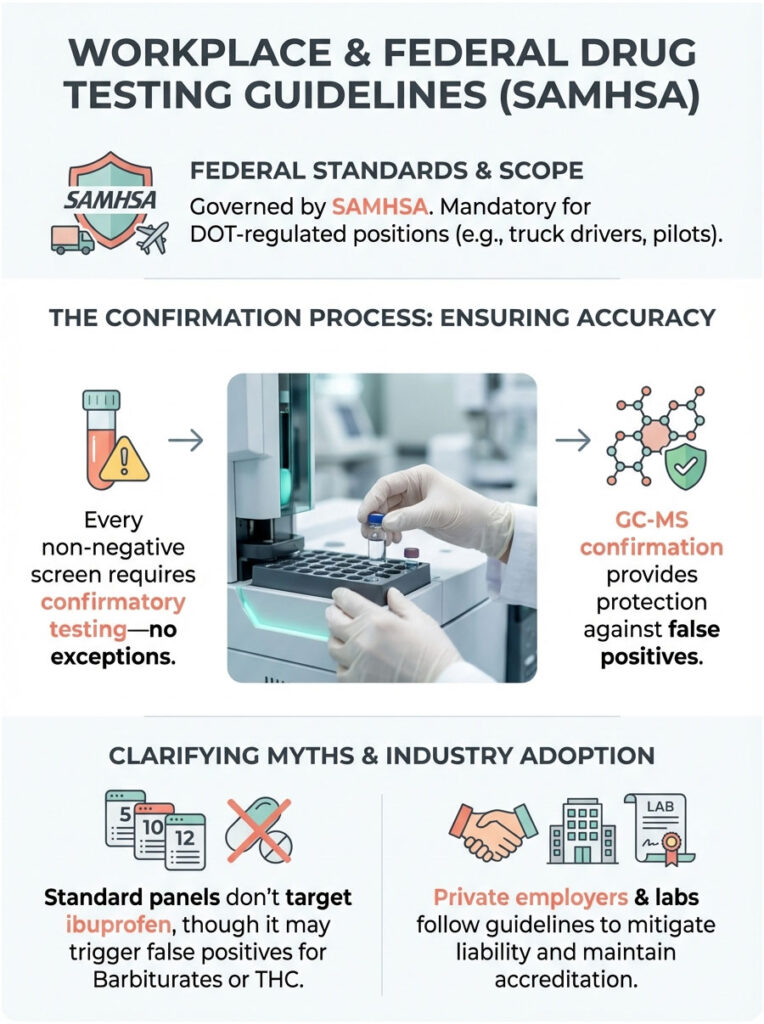
Federal vs. Private Sector Standards
If you are a truck driver, pilot, or hold any DOT-regulated position, your test follows federal guidelines. Under SAMHSA rules, every single non-negative screen must undergo confirmatory testing. No exceptions. If an initial screen flags for THC because of ibuprofen, the lab is legally required to run GC-MS.
The GC-MS identifies it as ibuprofen, the lab reports “Negative,” and the employer never even knows the screen was flagged. This protection exists specifically to prevent lawsuits and wrongful termination.
In the private sector, most employers follow the same guidelines to avoid liability, though they are not strictly mandated by federal law to the same degree. Reputable labs like LabCorp and Quest follow the SAMHSA model regardless of the client, largely to maintain their CAP and CLIA accreditation.
What the 5-Panel, 10-Panel, and 12-Panel Screens Cover
- 5-Panel: THC, Cocaine, Opiates, PCP, Amphetamines.
- 10-Panel: Adds Barbiturates, Benzodiazepines, Methadone, Propoxyphene, Quaaludes.
- 12-Panel: May add painkillers like Oxycodone or Ecstasy (MDMA).
Ibuprofen is not a target analyte on any of these. It is an OTC analgesic. The only time it becomes relevant is during a potential false positive for barbiturates or THC, as discussed above.
Home Drug Tests vs. Laboratory Tests: A Critical Difference
This is where a lot of people get into trouble. They buy a drug test kit from a pharmacy to “check” themselves before the real test. They take some Advil, test themselves, and see a faint ghost line. Panic follows.
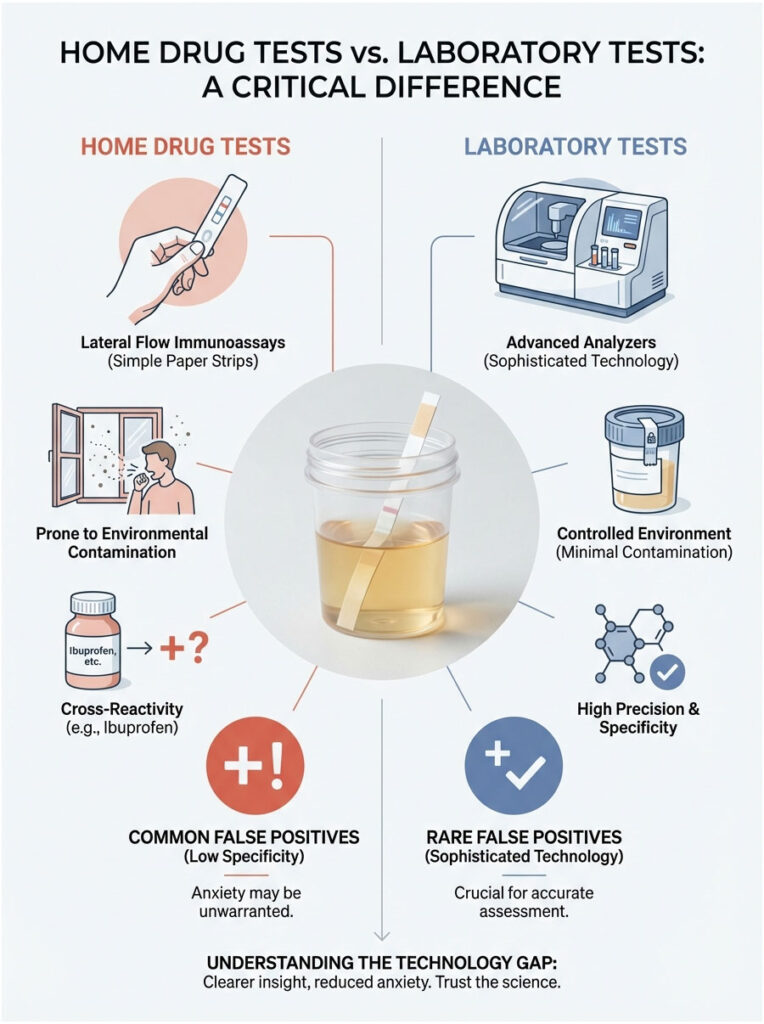
You need to understand the technology gap. Home tests are lateral flow immunoassays, basically simple paper strips coated with inexpensive antibodies. They lack the sophisticated filtering and pH balancing of laboratory analyzers. They are highly prone to environmental contamination and cross-reactivity.
A positive result on a home test caused by ibuprofen is actually quite common. But a positive result on a $50,000 Olympus analyzer at a toxicology lab is rare. Do not let a cheap test strip dictate your anxiety levels. Laboratory equipment operates in an entirely different league of precision.
Practical Tips for Test Takers
Even with the science on your side, you probably want to avoid the hassle of a flagged test entirely. Here is how.
Before the Test
Should you stop taking ibuprofen? Generally, no. But if you want zero anxiety, follow the “safe zone” rule: stop taking the medication 24 hours before your test. Given the short half-life, this clears the vast majority of metabolites.

Be smart about hydration. Some people drink enormous amounts of water trying to “flush” their system. This often backfires, leading to a “Negative Dilute” result because creatinine levels drop too low. Employers often view a negative dilute with suspicion and will order a retest. Just drink normal amounts of water.
Documentation and Disclosure
When you arrive at the collection site, you will fill out a chain of custody form. There is usually a space to list current medications. Does Advil cause a false positive drug test result? Rarely. But writing it down creates a paper trail that can help if questions come up later.
If you are taking prescription-strength ibuprofen (800mg), bring the bottle or a pharmacy receipt. While the lab technicians collecting the sample cannot use this information to alter results, it is useful for the MRO if a review becomes necessary.
How to Challenge a False Positive
In the incredibly unlikely event that a false positive slips through the system, you have rights.
- Demand the Confirmation: Ask specifically whether the result was confirmed via GC-MS. If it was not, the test is invalid by standard toxicological practices.
- Request the Split Sample: Federal guidelines require urine to be split into Bottle A and Bottle B. You have the right to have Bottle B tested at a different certified laboratory. That second test will clear you.
- Contact the MRO: Engage directly with the Medical Review Officer. Explain the situation and provide proof of your prescription or OTC usage.
How Dosage and Frequency Affect Your Risk
A single 200mg pill is chemically negligible in a large volume of urine. The cross-reactivity curves from reagent manufacturers are typically tested at much higher concentrations. To produce enough interference to fool a machine, the ibuprofen concentration in urine often needs to exceed 100,000 ng/mL.
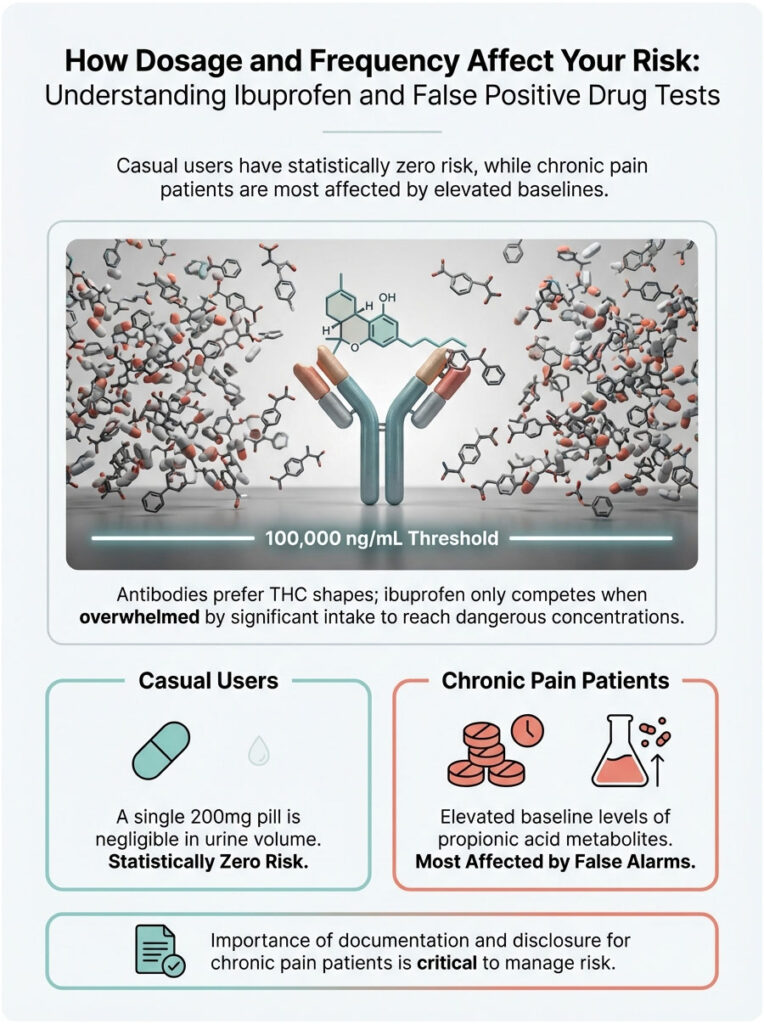
Reaching that level requires significant and sustained intake, meaning maximum daily doses taken over several days. If you are a casual user taking Advil for a hangover, your risk is statistically zero. The concentration simply is not high enough to compete with the antibodies, which strongly prefer the shape of THC. They will only grab ibuprofen if they are overwhelmed by it.
This is why chronic pain patients are the primary group affected by these false alarms. Their baseline levels of propionic acid metabolites are constantly elevated. If you fall into this category, the documentation and disclosure steps mentioned earlier become even more important.
Final Takeaway
So, will ibuprofen show up on a drug test? No. It is not a target drug. It will almost certainly never appear as a confirmed positive.
While ibuprofen false positive results on initial screens are a biological possibility due to immunoassay cross-reactivity, the safety net is strong. GC-MS confirmation eliminates the error. Modern toxicology can distinguish between the innocent use of a pain reliever and illicit substance use with high precision. The marijuana metabolite (11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid) is chemically distinct from ibuprofen metabolites at the molecular level.
Trust the process. If you have not taken illicit drugs, the confirmatory science is on your side. Between improved reagents, mandatory confirmation protocols, and MRO oversight, your headache medication stays exactly what it is: medicine, not a liability.
Frequently Asked Questions
Can taking ibuprofen cause a false positive for marijuana on a drug test?
Yes, it is biologically possible for ibuprofen to trigger a false positive for THC on initial immunoassay screens due to structural similarities in carboxylic acid metabolites. However, this cross-reactivity is extremely rare with modern FDA-cleared assays and is typically only seen with very high, chronic dosages. If a presumptive positive occurs, mandatory confirmatory testing will always distinguish the NSAID from illicit cannabinoids.
How long does ibuprofen stay in your system before a urine drug screen?
Ibuprofen has a relatively short half-life of approximately two hours, meaning about 99% of the drug is cleared from your system within 24 hours. For casual users taking standard over-the-counter doses, metabolites are usually undetectable or below interference levels within 12 to 18 hours. Chronic users or those with impaired renal function may retain metabolites slightly longer, potentially extending the detection window to 48 hours.
Will Advil or Motrin show up on a standard 10-panel drug test?
No, ibuprofen is not a controlled substance and is never a target analyte on standard workplace drug panels. These tests specifically look for illicit substances like cocaine, opiates, and amphetamines, or restricted prescription medications. While it may rarely interfere with the initial screening chemistry for other drug classes, it is never reported as a positive result for ibuprofen itself.
Why do some home drug tests flag ibuprofen as a positive result?
Pharmacy-grade home kits often utilize lower-quality lateral flow immunoassays that lack the sophisticated pH balancing and specialized antibodies found in clinical laboratory analyzers. These \”instant\” tests are much more susceptible to cross-reactivity and environmental factors, leading to higher rates of drug test interference. A presumptive positive on a home kit following Advil use is often a chemical error rather than a true detection of illicit drugs.
Can ibuprofen trigger a false positive for barbiturates?
Clinical evidence suggests that high concentrations of NSAIDs like ibuprofen and naproxen can occasionally cause cross-reactivity with barbiturate immunoassays. This occurs most frequently in patients managing chronic inflammatory conditions who maintain high serum levels of the medication. Fortunately, the specific molecular fingerprint of barbiturates is distinct, and confirmatory testing will quickly rule out any illicit substance use.
What is the purpose of GC-MS confirmation if I test positive after taking Advil?
Gas Chromatography-Mass Spectrometry (GC-MS) serves as the definitive gold standard to resolve any screening ambiguities caused by cross-reactivity. Unlike initial screens that look for general molecular shapes, GC-MS shatters molecules into fragments to identify their unique atomic mass. This process is 100% accurate in distinguishing ibuprofen metabolites from illegal substances like THC or barbiturates, ensuring a final Negative report.
Should I disclose my ibuprofen use to the lab technician at the collection site?
While the collection staff cannot interpret or change your results, you should document any recent medications on the provided chain of custody form. This creates a formal paper trail that a Medical Review Officer (MRO) can reference if a presumptive positive requires further investigation. Providing a prescription or receipt for high-strength Motrin can expedite the review process and reduce your anxiety during the testing window.
Does ibuprofen interfere with benzodiazepine results on a drug screen?
Generally, ibuprofen does not cross-react with the antibodies used to detect benzodiazepines like Xanax or Valium. While a different NSAID called Oxaprozin is known for this specific interference, ibuprofen\’s chemical structure is significantly different from the benzo ring. In rare cases of unique liver enzyme variations, unpredictable reactions can occur, but these are statistically negligible in a standard clinical setting.
How much ibuprofen is required to cause a drug test interference?
A single 200mg or 400mg dose is highly unlikely to reach the concentration levels—often exceeding 100,000 ng/mL—needed to overwhelm modern immunoassay reagents. Interference typically requires a loading effect, such as taking 800mg prescription doses multiple times a day for several days. Casual use for a headache or minor ache poses virtually zero risk of triggering a false alarm on professional laboratory equipment.
What role does a Medical Review Officer play if ibuprofen flags my test?
The Medical Review Officer (MRO) is a licensed physician who acts as an independent gatekeeper between the toxicology lab and your employer. If a screen flags for interference, the MRO reviews the GC-MS data; once they confirm the substance is merely ibuprofen, they report the final result as Negative. In most cases, the MRO and the lab resolve this internally, and the employer is never informed that a flag even occurred.
Can dehydration increase the risk of an ibuprofen false positive?
Yes, severe dehydration concentrates urine, which increases the specific gravity and the relative concentration of all metabolites, including those from NSAIDs. Higher concentrations of ibuprofen metabolites in a small volume of urine make it theoretically more likely to cross the sensitivity threshold of an initial immunoassay. Maintaining normal hydration levels ensures that your sample remains within standard physiological ranges, reducing the chance of chemical interference.
What are my rights if an employer claims I failed a drug test due to ibuprofen?
If an employer reports a failure based on a screen that you suspect was influenced by ibuprofen, you have the right to demand proof of GC-MS confirmation. Under SAMHSA and DOT guidelines, no employment action should be taken based solely on an initial immunoassay screen without molecular confirmation. You also have the right to request a split sample test, where a second independent lab analyzes your specimen to verify the original findings.
Disclaimer
This article is for informational purposes only and does not constitute medical or legal advice. While the information is based on clinical toxicology standards, individual metabolic factors and specific laboratory protocols may vary. Always consult with a qualified healthcare professional or legal expert regarding drug testing and employment rights.
References
- Substance Abuse and Mental Health Services Administration (SAMHSA) – Federal Drug Testing Programs – Provides official guidelines for federal workplace drug testing and confirmatory protocols.
- Journal of Analytical Toxicology – Oxford Academic – Peer-reviewed studies regarding immunoassay cross-reactivity and NSAID interference.
- Mayo Clinic Laboratories – Clinical & Interpretive Guide – Technical data on drug half-lives, detection windows, and GC-MS methodology.
- U.S. Department of Transportation (DOT) – Office of Drug and Alcohol Policy and Compliance – Regulations governing the role of the Medical Review Officer (MRO).
- Quest Diagnostics – Drug Testing Employer Solutions – Statistical data on annual workplace drug testing trends and common interference factors.
- National Center for Biotechnology Information (NCBI) – PubMed – Research papers on the molecular structure of ibuprofen metabolites and their interaction with EMIT reagents.